Ordering
Email: [email protected]
Ordering: +45 29 13 42 24
Main: +45 31 10 91 91
CVR/VAT no. 31348854
Technical Support
Email: [email protected]
Immudex’s products and components are classified as non-hazardous and therefore a Safety Data Sheet (SDS) is not required – please read our Non-hazard Classification Statement
However, we strongly recommend using prudent laboratory practices: avoiding unnecessary contact, and use of personal protective equipment, which may include chemical resistant gloves, eye protection, and lab coats, during the use of laboratory reagents
| Symbol | Title of symbol |
Explanatory Text | Reference |
 |
CE marking of European Conformity | Indicates that the product is in conformity with applicable European regulations |
IVDD 98/79/EC Annex X |
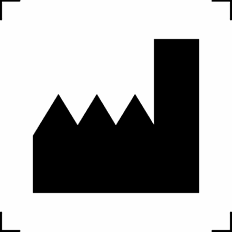 |
Manufacturer | Indicates the product manufacturer |
ISO 15223-1:2016 Reference no. 5.1.1 |
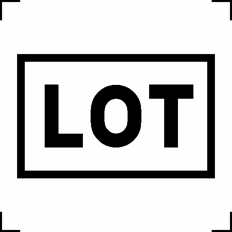 |
Batch code | Indicates the manufacturer’s batch code so that the batch or lot can be identified |
ISO 15223-1:2016 Reference no. 5.1.5 |
 |
Catalog number | Indicates the manufacturer’s cataloge number so that the product can be identified |
ISO 15223-1:2016 Reference no. 5.1.6 |
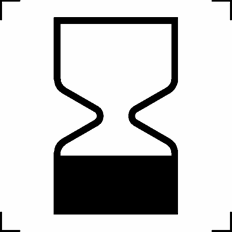 |
Use-by date | Indicates the date after which the product is not to be used |
ISO 15223-1:2016 Reference no. 5.1.4 |
 |
In vitro diagnostic medical device | Indicates a product that is intended to be used as an in vitro diagnostic medical device |
ISO 15223-1:2016 Reference no. 5.5.1 |
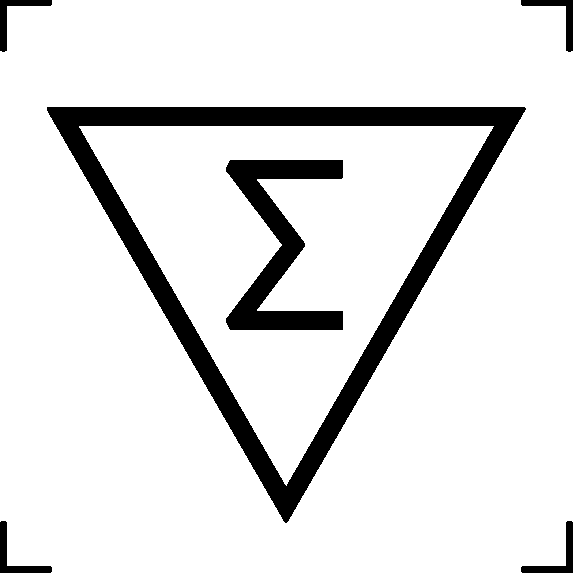 |
Contains sufficient for <n> tests | Indicates the total number of tests that can be performed with the device |
ISO 15223-1:2016 Reference no. 5.5.5 |
 |
Serial number | Indicates the manufacturer’s serial number so that a specific product can be identified |
ISO 15223-1:2016 Reference no. 5.1.7 |
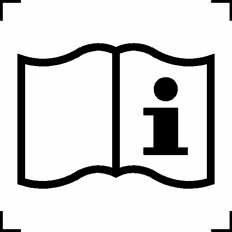 |
Consult instructions for use | Indicates the need for the user to consult the instructions for use |
ISO 15223-1:2016 Reference no. 5.4.3 |
 |
Temperature limit for storage in the dark | Indicates the temperature limits to which the product can be safely exposed, and that the product must be kept in the dark until use | Modified from ISO 15223-1:2016 |
 |
Research use only | Indicates that a product is intended for research use; not for use in diagnostic procedures | 21 CFR 809.10 (c) (i) |
 |
Good manufacturing practice | Indicates that the product is manufactured according to quality system requirements | 21 CFR 820 ISO 13485:2016 |
 |
Analyte specific reagent | Caution: Analytical and performance characteristics are not established | 21 CFR 809 (e) |
 |
Custom Solution & Service |
Developed and/or manufactured according to customer specifications |
Immudex generated symbol |
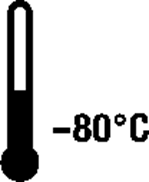 |
Storage temperature | Indicates the temperature to which the product should be stored | Modified from ISO 15223-1:2016 |
 |
Keep cool at all times | Indicates the temperature to which the product should be kept at all time | Immudex generated symbol |
 |
Prescription use only | Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner | 21 CFR 801.109 |
Ordering
Email: [email protected]
Ordering: +45 29 13 42 24
Main: +45 31 10 91 91
CVR/VAT no. 31348854
Technical Support
Email: [email protected]