- Introduction to Potency Assays for TCR-T Cell Therapies
- Cytokine production following stimulation with Xynapse™-T - IFN-γ, TNF-α, and IL-2
- Enrichment of TCR-engineered T cells following stimulation with Xynapse™-T
- Biomarker expression patterns of TCR-engineered T cells activated with Xynapse™-T
- Evaluation of Xynapse™-T for a second TCR-T cell therapy product
Potency Testing of TCR-T Cell Therapies
Potency Assays Required to Evaluate TCR-T Cell Therapies
T cell receptor therapy has great promise for the treatment of solid tumors and in 2024 the U.S. FDA approved the first therapy for solid tumors based on TCR engineered T cells derived from the patient’s own T cells. The therapy named afami-cel is approved for the treatment of advanced synovial sarcoma and it is hopefully only the first example of many novel therapies to come.
Multiple T cell receptor therapies are currently in development for treatment of other cancer indications. Quality control of autologous cell therapy drug products derived from the patient’s own immune cells presents unique challenges distinct from other drug modalities. This is due to the complexity of living cells with multiple effector functions and the variable nature of patient-specific starting material.
An important part of the successful launch of a new cell therapy is the development of validated potency assays for release testing of cell products. These assays measure biological activities of the drug product which, per U.S. FDA guidance, must accurately reflect the mechanisms of action of the product to demonstrate its efficacy and safety1 and ideally correlate with clinical response.
The Immune Synapse at the Heart of Potency Assays
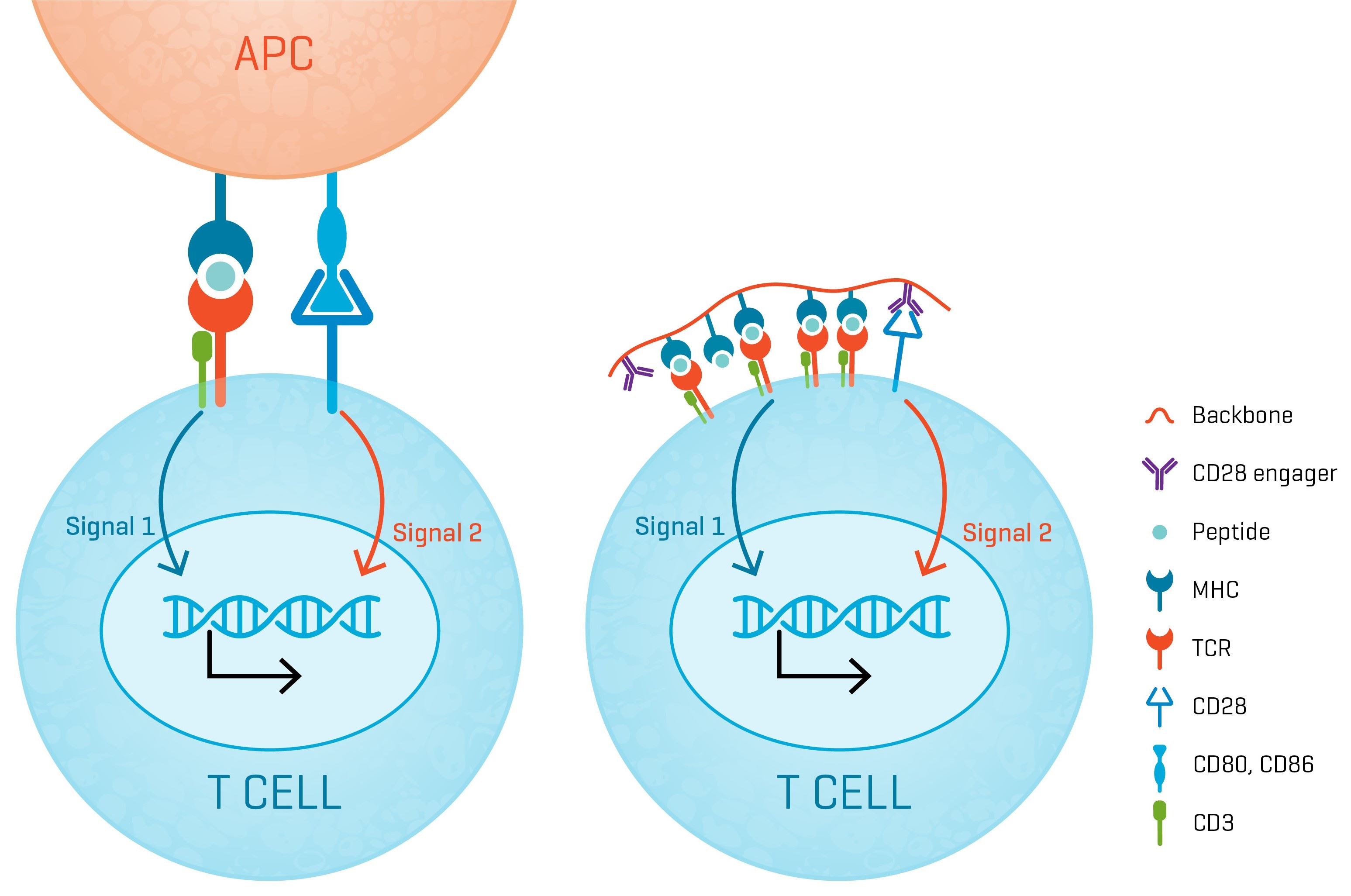
T cell receptor therapy is based on engineered T cells with TCRs designed to recognize a cancer specific epitope presented by a defined HLA. Thus endowed, the TCR-T cells are able seek out cancer cells presenting the cognate pMHC and kill them. Central to this activity is the formation of immune synapses between the TCR-T cells and antigen presenting cells (APC) and cancer cells which will trigger activation of the TCR-T cells leading cytokine secretion, clonal expansion and cytotoxic activity towards cancer cells specifically.
For this reason, potency assays for TCR-T cell products typically monitor the downstream effects of immune synapse formation in vitro between the TCR-T cells and antigen-presenting cells (APCs) loaded with the cognate peptide epitope. During drug development multiple assays are used to assess T cell activation, such as cytokine production assays, T cell expansion and cytotoxic activity. These biological effects are correlated and often a single or few surrogate markers can be selected for potency testing of commercially produced drug products (typically IFN-γ production).
APC-Derived Biological Variability
However, assays based on living APCs are intrinsically associated with high variability. In addition, their preparation and γ maintenance are labor-intensive, including cell culture, peptide loading, and validation. Furthermore, variable cell conditions, proliferation rates and risk of infections lead to unpredictable timelines. Delays in product release testing that can result from that inconsistency are critical for the terminally ill patients waiting for their treatment.
A Robust Mimic of the Immune Synapse
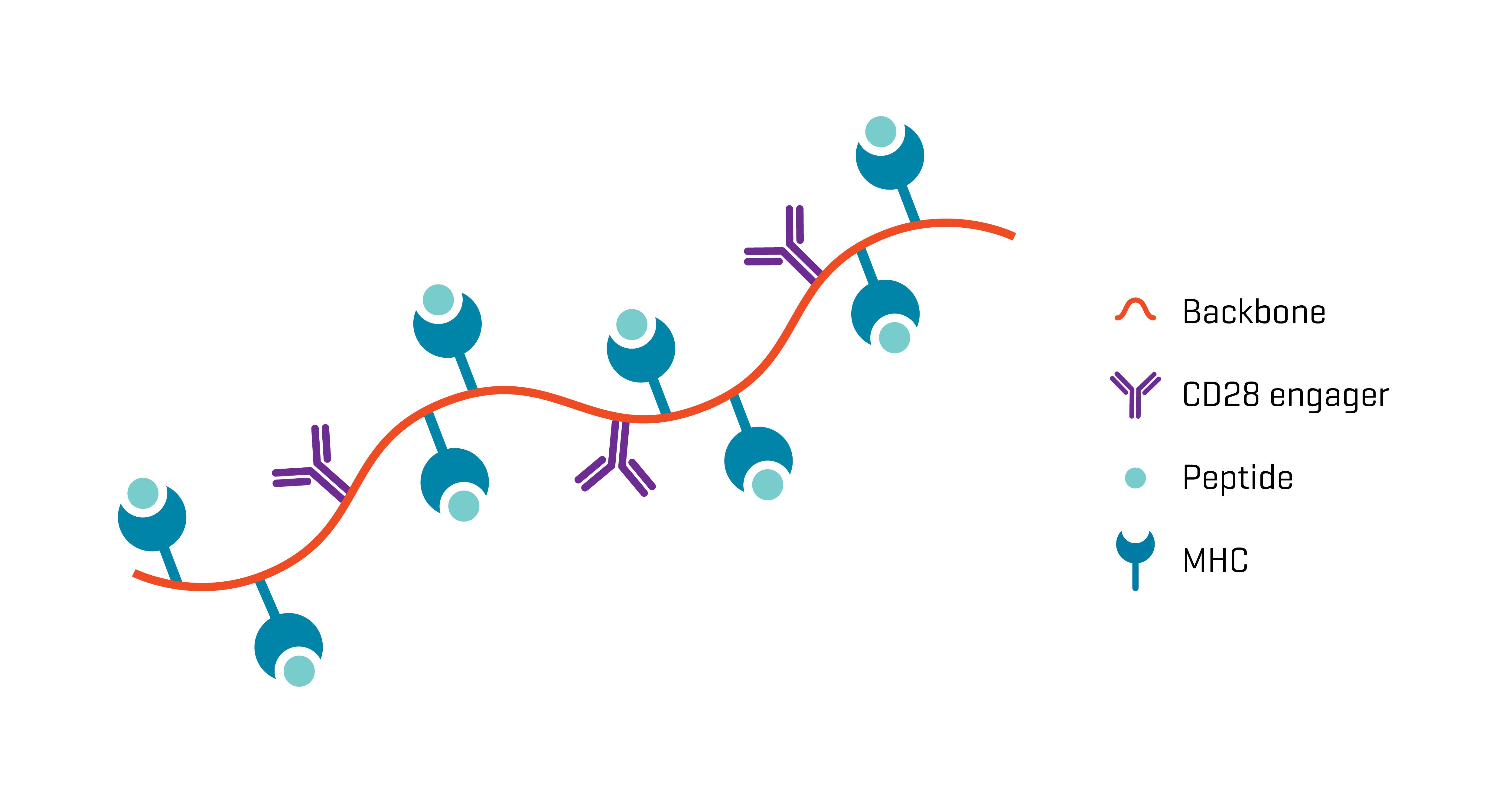
XynapseTM-T reagents are synthetic antigen-presenting molecules that eliminate the variability of working with APCs, offering significant time, labor, and standardization advantages. They consist of a polymeric backbone presenting peptide-MHC complexes and CD28 engagers to mimic the two-signal T cell activation by APCs.
Faster and More Flexible Potency Assays using Xynapse™-T Reagents
Ready-to-use XynapseTM-T reagents are produced rapidly and can be ordered with the peptide-MHC complex of choice, enabling design of highly standardized antigen-challenge assays.
Substituting APCs with synthetic XynapseTM-T molecules offers great flexibility in the planning and fast execution of potency assays, while the uniformity in performance ensures that results are reliable and comparable.
Are Xynapse™-T Reagents a Reliable Substitute for APCs?
To serve as proxy for peptide-pulsed APCs, XynapseTM-T reagents must demonstrably mimic their activation power. The following data were collected in collaboration with a partner comparing XynapseTM-T reagents with their APC counterpart and an αCD3 & αCD28 T cell activation reagent.
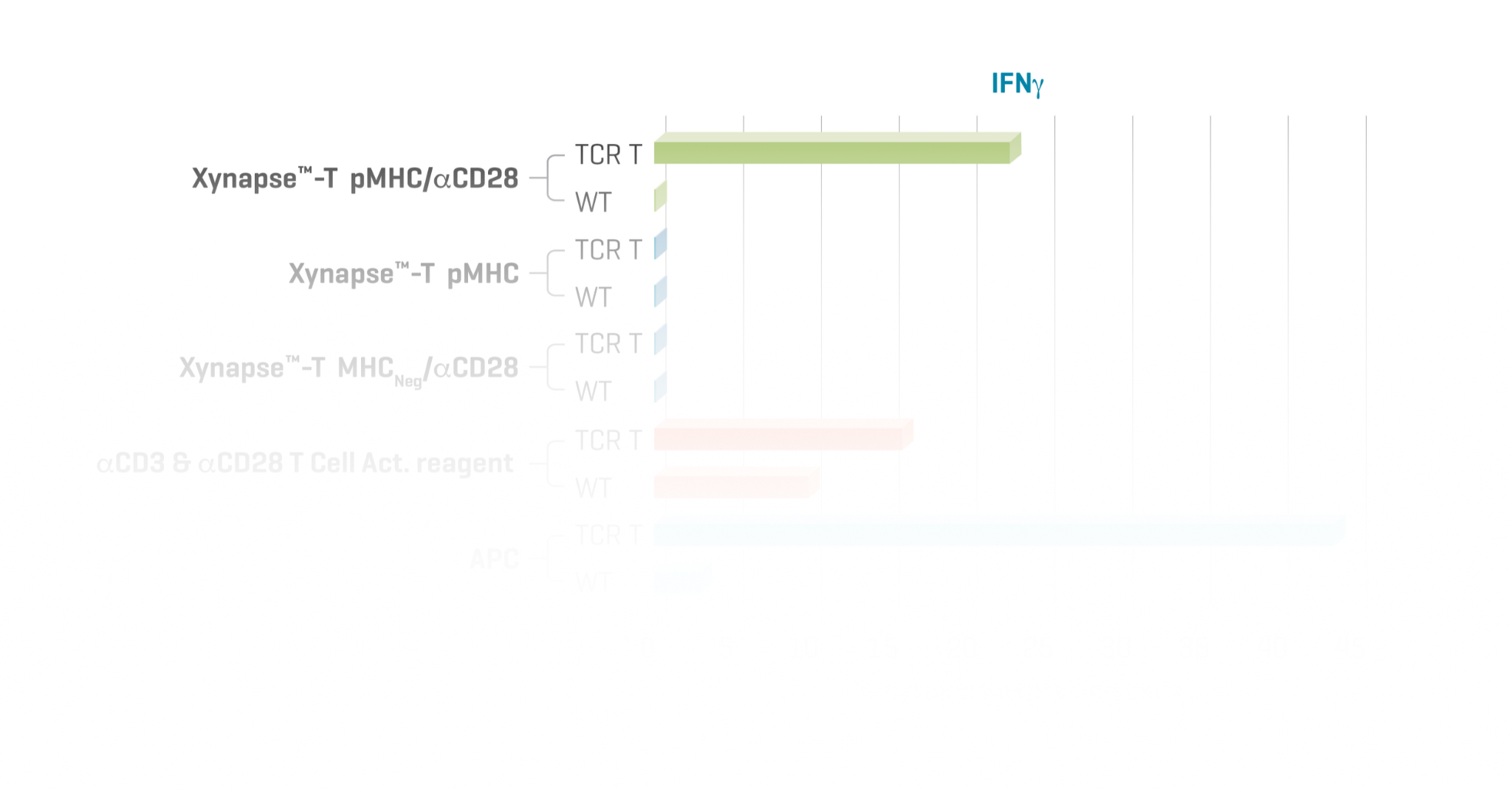
Stimulation of TCR-Engineered T Cells with XynapseTM-T Reagents is Antigen-Specific and αCD28-Dependent
TCR-engineered T cells with specificity for a defined cancer epitope and their wild type parental cells were co-cultured with XynapseTM-T reagents including various controls, an αCD3 & αCD28 T cell activation reagent, or APCs pulsed with the cancer epitope. After a 6-hour incubation, the proportion of TCR-engineered T cells producing the cytokines IFN-γ, TNF-α, and IL-2 were measured by flow cytometry.
To continue reading this blog, please fill in your details below.
