MHC Multimer Proficiency Panel Report 2020
MHC Multimer Proficiency Panel Report 2019
MHC Multimer Proficiency Panel Report 2018
Originally developed at the initiative of CIC (the US Cancer Immuno-therapy Consortium of the CRI) and CIMT (the European Association for Cancer Immunotherapy), Immudex offers Proficiency Testing as a non-profit service to help researchers and clinicians worldwide evaluate and benchmark their immune monitoring performance with MHC Multimers.
Using laboratory-specific protocols, antibodies, and flow cytometry, participants determine the number of antigen-specific T cells in provided PBMC samples using MHC multimers and receive a report comparing the results from all participating laboratories.
Quality controlled PBMC samples – identical for all participants
If requested, MHC Dextramer® reagents free of charge
Assay instructions
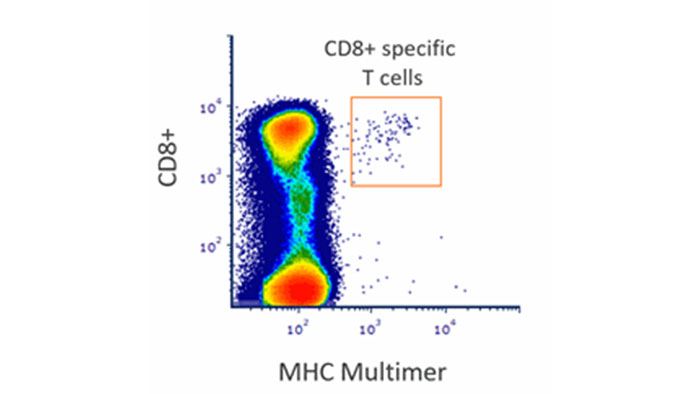
Total number of CD8+ T cells in samples
Number of antigen specific CD8+ T cells in samples, stained with MHC Multimers
In addition, as a special feature this year, we offer participants the option of enumerating MAIT cells with our new MR1 Dextramer®
| SIGN UP | RECEIVE SAMPLES | ANALYZE SAMPLES | UPLOAD DATA | RECEIVE REPORT |
 |
 |
 |
 |
 |
| Register | PBMCs MHC Dextramer® if requested Assay instructions |
Use your own protocol to analyze PBMC samples according to the instructions | Report your results back to Immudex | Get a report with all participants' performance |
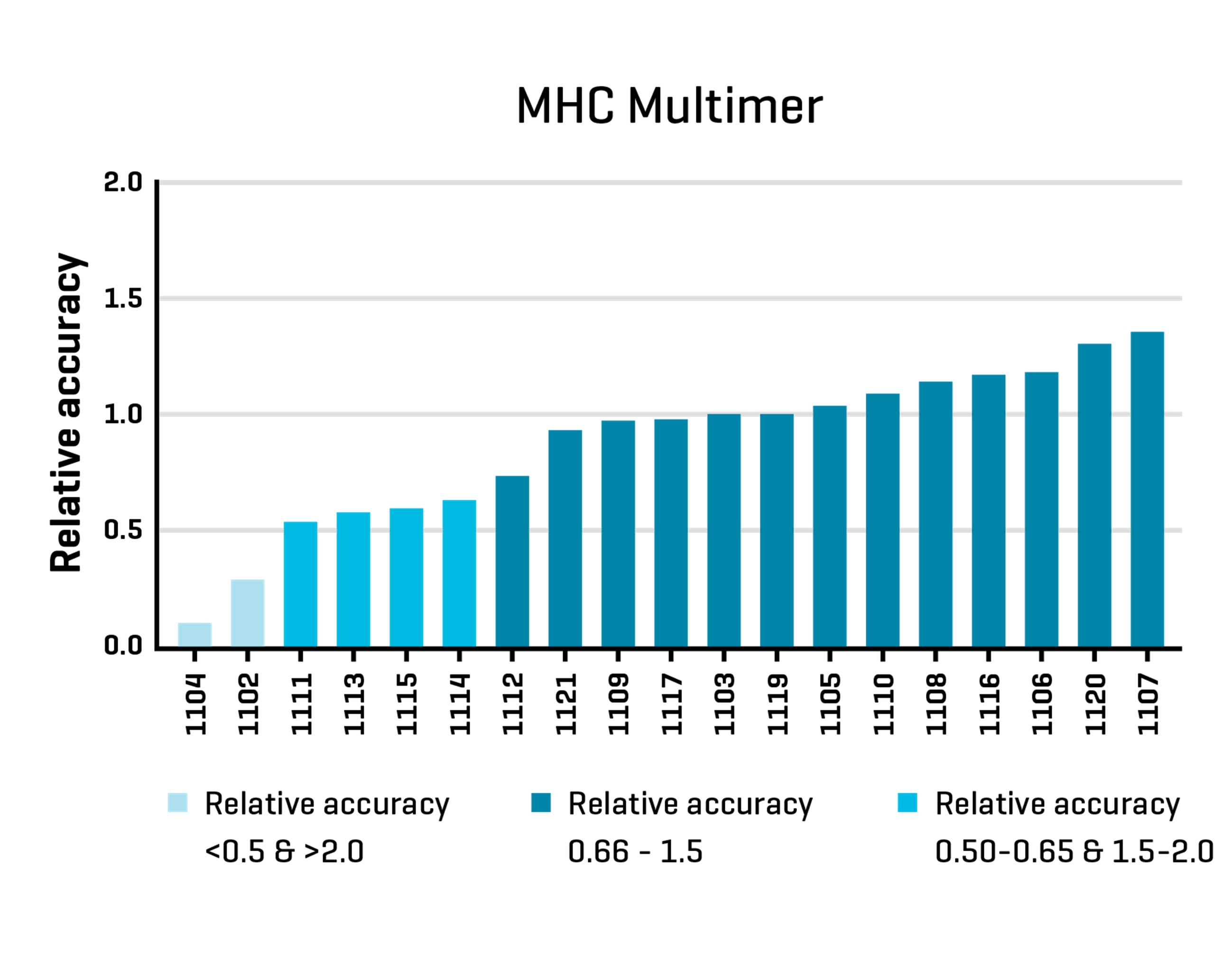
In the MHC Multimer Proficiency Panel 2020, all laboratories received identical PBMC samples for identification of antigen-specific T-cells using MHC multimers, and results were collated to assess the relative accuracy of results across the different laboratories. 13 out of 19 participants (68,4%) had a relative accuracy between 0.66 – 1.5 and were considered to be in “the average range” (dark blue columns).
Learn more in the MHC Multimer Proficiency Panel Report 2020
The following MHC monomers are used in this years MHC Multimer proficiency testing. We provide Dextramer® reagents free of charge upon request, but participants are welcome to use multimers of their choice with the below specified monomers.
|
Allele |
Specificity |
Antigen |
Category |
|
HLA-B*0801 |
FLRGRAYGL |
EBNA 3A |
EBV |
|
HLA-B*0801 |
RAKFKQLL |
BZLF1 |
EBV |
|
HLA-A*0801 |
|
|
Negative control |
|
MR1 (human) |
5-OP-RU* |
|
MAIT activating ligand |
|
MR1 (human) |
6-FP |
|
Negative control |
*For detection of MAIT cells