- In a phase I clinical trial, Hong, D.S. et al. (2023) evaluated the safety and efficacy of Adaptimmune’s autologous MAGE-A4-directed TCR-T cell therapy product (afami-cel). The study used MHC Dextramer® reagents to assess transduction and to track the kinetics of afami-cel persistence in post-infusion PBMC samples. TECELRA® (afami-cel) is the first TCR-T cell therapy for solid tumors to be FDA approved (see press release).
- In a First-in-Human dose escalation trial, Wermke et al. (2024) evaluated the clinical safety and efficacy of Immatics’ autologous MAGEA1-directed TCR-T cell therapy product (IMA202). The study used MHC Dextramer® reagents to quantify MAGEA1-specific T cells for dose determination. IMA202 T cells persisted in peripheral blood for several weeks to months and were detected in tumor tissue.
- Foy, S. et al. 2022 used CRISPR-Cas9 non-viral genome editing to simultaneously knock out endogenous TCR genes and insert sequences of a neoantigen-specific TCR taken from circulating T cells of patients. Dextramer® reagents were used to confirm surface expression and specific binding of the generated novel TCR.
- A phase I/II clinical trial demonstrated safety, targeted trafficking, and extended persistence of an autologous TCR T-cell therapy to treat multiple myeloma. Rapoport, A.P. et al. (2015) engineered T cells to express an affinity-enhanced TCR to recognize a peptide shared by NY-ESO-1 and LAGE-1. They used Dextramer® reagents in immunohistochemistry and flow cytometry to detect and track the engineered T cells in blood and bone marrow tissue for two years after infusion.
Release Testing of TCR-T Cell Therapies
Must-test parameters to release a TCR-T cell therapy
T cell receptor (TCR)-T cell therapies are a form of adoptive cellular therapy (ACT) that uses genetically modified lymphocytes to target specific tumor antigens presented by major histocompatibility complexes (MHC). Unlike chimeric antigen receptor (CAR)-T cell therapies, which recognize surface antigens, TCR-T cell therapies can target antigens derived from any cellular compartment, including intracellular proteins and neoantigens. This expands the range of potential targets for cancer immunotherapy, especially for solid tumors.
Developing and manufacturing TCR-T cell therapies is a complex process with rigorous quality control. Release testing is performed on the final cell product before infusion into patients to ensure that it meets various predefined specifications for quality, safety, and efficacy. The battery of tests performed varies depending on the nature of the cell product but commonly includes, on the one hand, standard assays for sterility and the absence of endotoxins and mycoplasma, and on the other hand, multiparametric flow cytometry to ascertain T-cell identity.
Accurately counting the number of antigen-specific T cells in a lot and confirming the correct specificity of T cells in the product (or balance of specificities in the case of combination therapies) is critical. The performance of TCR-T cell therapies hinges on an optimal and clinically significant number of therapeutically functional T cells, given that a limited number of cells can be infused without side effects, and consistency of product lots is needed to ensure that variation in clinical outcomes is not due to differences in administered doses.
The safety of TCR-T cell therapies and related risk mitigation are top priorities of regulatory bodies and release testing is a key aspect of that mitigation. Table 1 summarizes FDA guidelines1 for release testing and other safety-related evaluations. The latter include in vitro specificity and phenotyping studies and characterizing on/off-target and on/off-tumor effects.
FDA guidelines1 |
Corresponding Immudex solution |
|
Release testing:
|
Determine the of % Dextramer®-positive cells by flow cytometry (Fig. 1)
|
|
Pre-clinical in vitro specificity and characterization |
|
|
Specificity:
|
|
FDA guidelines1, as we understand them, emphasize the importance of using evaluation methods with documented high-quality assay components that adequately permit safety assessment. They also encourage doing so early in development to prevent costly changes later and to facilitate the transition from research and development to industrial manufacturing where appropriate product characterization is critical. Our MHC I Dextramer® (GMP) reagents provide the quality assurance necessary for lot release testing of TCR-T cell therapies (see sidebox for selected published studies). The reagents meet all criteria in terms of quality, as well as having an established shelf-life, stated expiry date, and a certificate of analysis.
“The FDA recommended that we use a GMP-grade multimer for the purposes of lot-release testing our TCR-T based therapy.”
Immudex customer, Scientist at a pharmaceutical company
TCR-T cell therapies must meet strict and narrow criteria to ensure safety, quality, and efficacy. With MHC I Dextramer® (GMP) reagents you can reliably ascertain the performance of your cell product for clinical use.
Release Testing using MHC Dextramer®
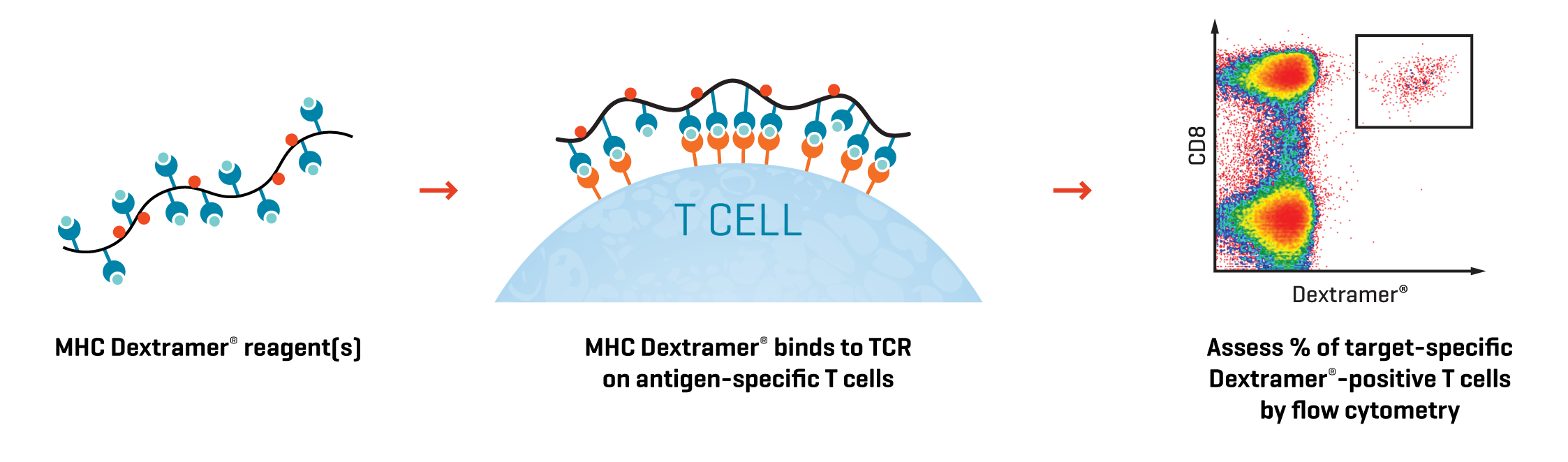
Fig. 1. Release testing of the TCR-T infusion product. MHC I Dextramer® (GMP) reagents can help to assess transduction efficiency, functionality and pre-infusion phenotype.
“Immatics uses Dextramer® reagents from Immudex for lot-release testing of TCR-based cell therapy product candidates. Being able to source high-quality pMHC multimer reagents from a reliable provider is important to us.”
Kerry Sieger, M.A., Senior Director Global Quality Operations, Immatics
References
1. CMC considerations for manufacturing gene modified T cell products. Presentation at the CAR-TCR Europe Summit March 2023 by Alan Baer, PhD, Staff Fellow, Division of Cellular & Gene Therapies, Center for Biologics Evaluation and Research (CBER), U.S. Food and Drug Administration (FDA).
Related Resources
Cell Therapy
Explore how Dextramer® reagents support the development of effective cell therapies.
TCR Webinar
Hear about the latest tools and techniques to discover and validate TCR candidates.
MHC I Dextramer® (GMP) Reagents
For applications that require reagents of the highest quality.

