Sensitively quantify target immune cells by flow cytometry with a strong signal-to-noise ratio.
Unraveling Patient Responses to Checkpoint Inhibitors
Tumor mutation burden (TMB), a measure of the number of genetic mutations in a tumor, has gained traction as an important determinant of patient response to checkpoint inhibitor (CPI) therapy. The Precision Medicine Working Group of the European Society for Medical Oncology (ESMO) recommends measuring TMB in “cervical cancer, well- and moderately-differentiated neuroendocrine tumors, salivary cancers, thyroid cancers, and vulvar cancers, as TMB-high predicted response to pembrolizumab in these cancers".i In this post, we dive into two studies exploring the idea that what connects a high TMB with a positive response to CPI therapy is an increased number of neoantigens that activates more T cells. We suggest that understanding the relationship between TMB and CPI therapy success requires monitoring all neoantigen-specific T cell responses, including subpopulations with rare or low-affinity T-cell receptors (TCRs) that are sensitively detected using Dextramer® technology but may go undetected by other multimers such as tetramers.
TMB versus CPI: the Connection
Studies in melanoma, non-small cell lung, colon, and uterine cancer show a correlation between TMB and response to CPI therapy.ii An analysis of 27 tumor types found that the correlation extends across other cancer types. The objective response rate to a CPI blockade was higher in tumor types with a higher mean number of coding somatic mutations per megabase of DNA. However, the authors highlight that the linear correlation coefficient of 0.74 accounts for only 55% of the differences across cancer types.iii There are often ‘exceptions to the rule,’ like Merkel -cell carcinoma, which responds better to CPI therapy than expected, and colorectal cancer with mismatched repair proficiency, which responds worse than predicted by TMB.
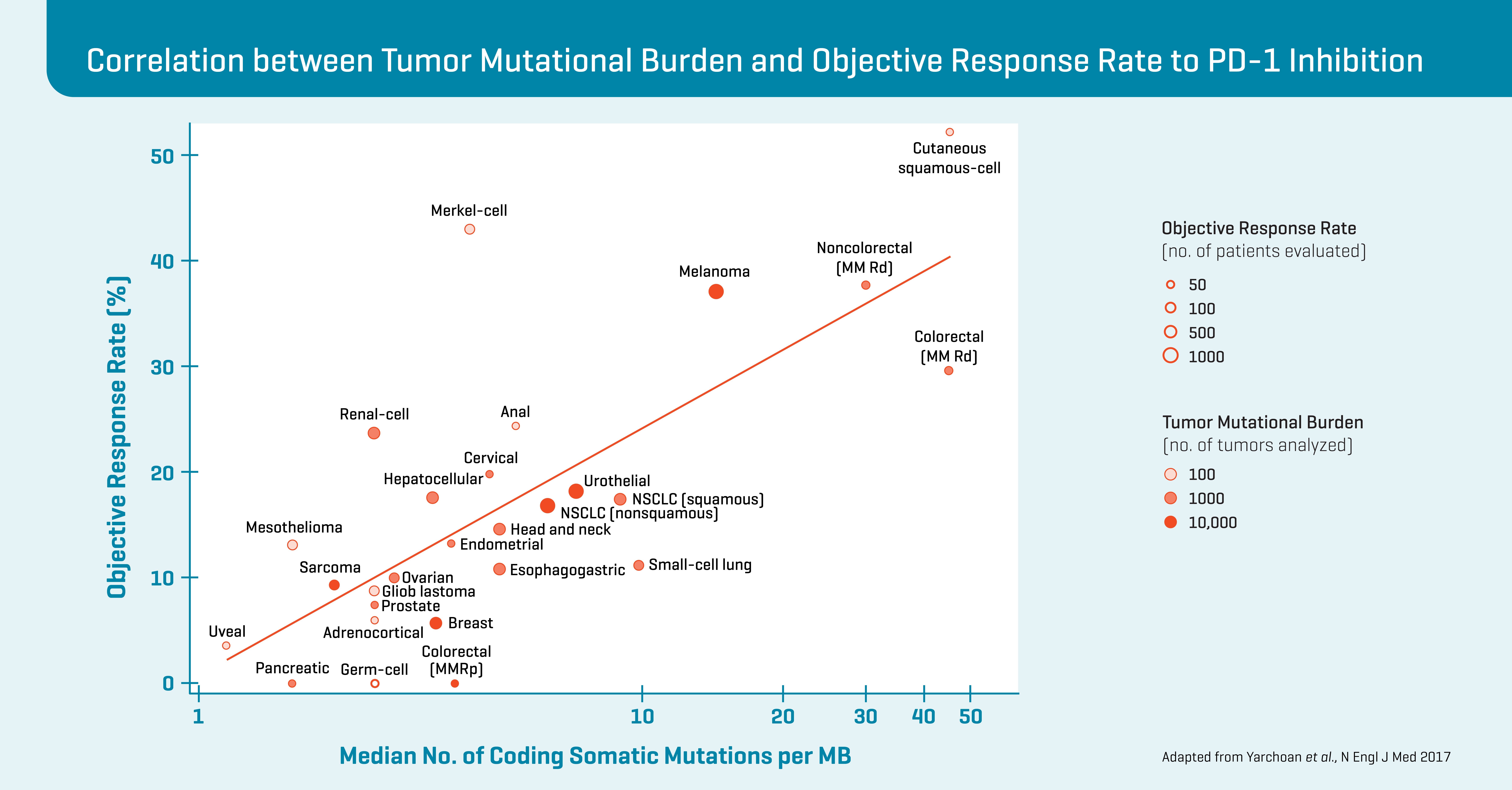
Adapted from Yarchoan et al., N Engl J Med 20173
Something beyond TMB determines patient response to CPI therapy. In the early 2010s, research groups began exploring neoantigens as the connection between TMB and CPI therapy response: “The mechanistic link between high mutation load and response to checkpoint blockade involves an increase in the number of neoantigens as mutation numbers rise.”ii Perhaps details about the steps linking more mutations to more neoantigens and thus greater T cell recognition help clarify CPI therapy responses. In fact, a model that included the likelihood of neoantigen presentation on MHC and the likelihood of T cell recognition in addition to TMB was better at predicting survival in patients who had undergone CPI treatment.iv
Sensitive T cell Detection likely Reveals More
In 2016, McGranahan and colleagues examined the role of clonal neoantigens versus subclonal neoantigens in determining sensitivity to CPI therapy.v Examining early-stage and late-stage lung cancer tissues from patients who had been treated with CPI, they found that a high neoantigen burden was associated with longer overall survival. However, they also found that tumors with high neoantigen burden were more homogeneous, suggesting the importance of clonality. So, they used MHC multimers to detect the presence of T cells reactive to hundreds of predicted neoantigens, screening mostly clonal neoantigens – those present in all tumor cells – but also over 250 subclonal ones present in subsets of tumor cells. They were only able to detect T cells that recognized clonal antigens. They concluded that clonal neoantigens drive more effective antitumor immunity than subclonal neoantigens, but interestingly then concede that a “higher neoantigen intratumoral heterogeneity may result in lower antigen dosage as compared with homogeneous tumors with high clonal neoantigen burden, thus reducing the chances of identifying T cells reactive to subclonal neoantigens.” In other words, it’s possible that T cell populations reactive to subclonal neoantigens, which are less dominant, may have gone undetected.
Higher Order Multimer Technology
The risk that the sensitivity of reagents may limit detecting heterogeneity in neoantigen-specific T cell populations was an important consideration in a study by Holm and colleagues.vi The authors used DNA-barcoded MHC multimers loaded with neoantigens predicted for each of 24 urethral carcinoma patients to examine the presence of neoantigen-recognizing T cells in peripheral blood. They showed that patients who responded to CPI therapy exhibited a “broadening in the number of neoantigen-recognizing T cell populations" 3 weeks after treatment compared to pre-treatment. Interestingly, they found no direct association between the number of unique neoantigen-recognizing T cell responses detected and the number of neoantigens predicted and evaluated for each patient. Thus, more than just TMB appears to influence responses during CPI treatment. In their work, Holm and colleagues also used tetramers to track 65 neoantigen-recognizing T cell responses in patients beyond 3 weeks. They note that 9 of the 65 responses they monitored were barely detectable at 9 weeks and comment that “borderline detectable responses may represent low-affinity neoantigen recognizing T cells, likely detected only by using DNA barcode-labelled multimers due to enhanced sensitivity compared to conventional tetramer-based detection of such T cells.”
How long do neoantigen-recognizing T cells that expand in CPI therapy persistent? What determines which T cell populations respond to CPI therapy? Might subclonal neoantigens play a more important role in response to CPI therapy than evidence to date suggests? Given the complexity of the cellular response to CPI, using highly sensitive MHC Dextramer® or dCODE Dextramer® reagents for a nuanced characterization of T cell specificity – even rare or low-affinity ones – is likely pivotal to see the full picture.
dCODE Dextramer® Reagents
Gold standard MHC multimers for analysis of antigen-specific T cells by NGS or single-cell multi-omics.

