Get the case study as a PDF.
Cancer Neoepitopes for Immunotherapy: Developing Strategies for Adoptive T-Cell Therapy
Background
Tumor-infiltrating lymphocytes (TILs) can recognize shared tumor-associated antigens (TAAs) and mutation-derived neoepitopes specific to the tumor or individual patient. Therefore, adoptive T-cell therapy based on one of these features of TILs is an attractive prospect for cancer treatment.
This study highlights the challenges of activating autologous TILs by patients’ neoepitopes and suggests strategies for better neoantigen-targeted immunotherapy.
Study Description
Goal: to study immunogenic neoepitopes using two distinct strategies: peptide reactivity of T cells isolated from the tumor and mass spectrometry detection of neoepitopes presented on the surface of the tumor cells:
- Exome sequencing and epitope prediction were performed from tumor cell lines from two HLA-A2*0201 melanoma patients (KADA and ANRU) with strong tumor reactivity of TILs
- Activation of TILs was analyzed by IFN-γ ELISA or flow cytometry
- Recognition of melanoma TAAs and neoepitopes by TILs was assessed using either MHC Dextramer® Melanoma Panel or neoepitope-specific MHC I Dextramer® staining by flow cytometry (Fig.1.).
Results
- TILs recognized 5/181 and 3/49 of the predicted neoepitopes
- TILs were unable to recognize the MS-defined neoepitopes AGPS and ENC1 detected on tumor cells of KADA
- In KADA, TILs were detected to be specific for KDELR2, MYLIP, and SVIL epitopes, but not for WDR75
- In ANRU, all 3 predicted neoepitope-specific TILs (ETV6 9mer, ETV6, 10mer, and NUP210) resulted in well-defined populations, comparable to the staining of MART-1.
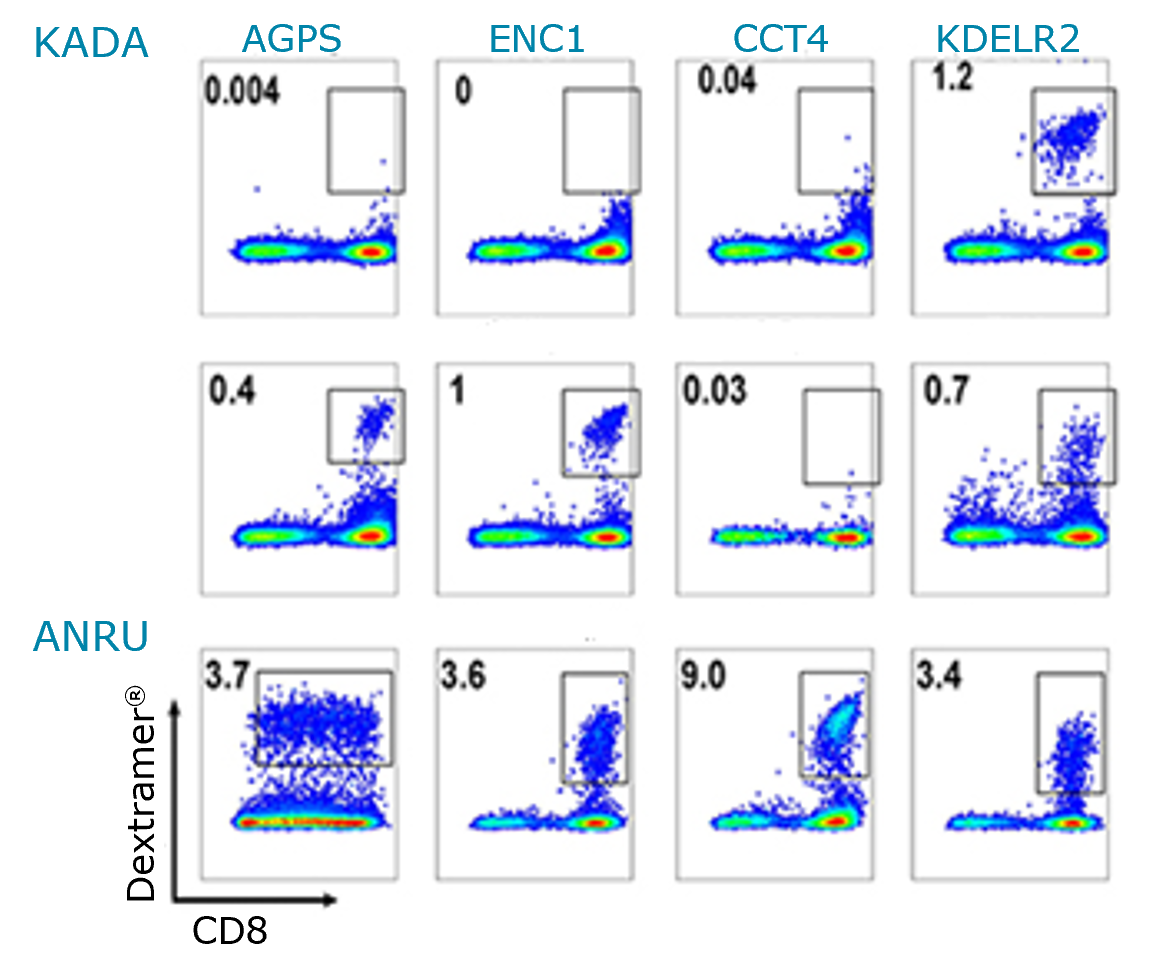
Fig.1. Detection of activated TILs was assessed by staining with the neoepitope-specific MHC I Dextramer® reagents. The MHC Dextramer® Melanoma Panel was used for staining MART-1-specific TILs by flow cytometry. FLU-specific Dextramer® was served as a positive control.
Conclusions
- The recognition of neoepitopes is highly specific, and tolerance to wild-type antigens has not been broken since they could not activate TILs
- The ability of TILs to recognize autologous tumor cells can be efficiently assessed using either Melanoma Dextramer® Panel that recognizes shared TAAs or neoepitope-specific MHC I Dextramer® reagents for mutation-derived neoepitopes.

