Get the case study as a PDF.
An RNA Vaccine Drives Immunity in Checkpoint-Inhibitor-Treated Melanoma
Background
Cancer immunotherapy targeting tumor-associated antigens (TAAs) is conceptually attractive, but relatively weak immunity is observed in patients with advanced cancer. RNA cancer vaccines targeting patients' TAAs can help to overcome this challenge.
This study highlights the first-in-human phase I clinical trial (Lipo-MERIT, NCT02410733) of the liposomal RNA vaccine FixVac (BNT111) in patients with advanced melanoma, including CPI-experienced patients.
Study Description
Goal: evaluate the treatment efficacy and patients’ immunity following vaccination.
89 patients expressing at least one of the four non-mutated TAAs targeted by melanoma FixVac have been vaccinated with FixVac alone or in combination with anti-PD1 immune checkpoint inhibitor therapy followed by optional continued monthly vaccination.
Antigen-specific CD8+ T-cell responses against melanoma-specific epitopes were analyzed using MHC I Dextramer® reagents and flow cytometry. Immune monitoring was performed for 49 patients, including phenotypic characterization of T-cell responses.
Results
- De novo induced CD8+ T-cells identified by MHC I Dextramer® analysis were of the PD1+CCR7-CD27+/-CD45RA- effector memory phenotype
- In patients with continued vaccination, the pool of circulating memory T-cells (particularly, NY-ESO-1- and MAGE-A3-specific CD8+ T cells) was continuously increasing in frequency or stayed stable over more than one year (Fig. 1.)
- In patients without maintained vaccination, TAA-specific CD8+ T cells were detected over a few months, with a gradual decrease in kinetics afterward
- >35% of the tumor-regression rate was detected in FixVac/anti-PD1 combination therapy in CPI-experienced patients
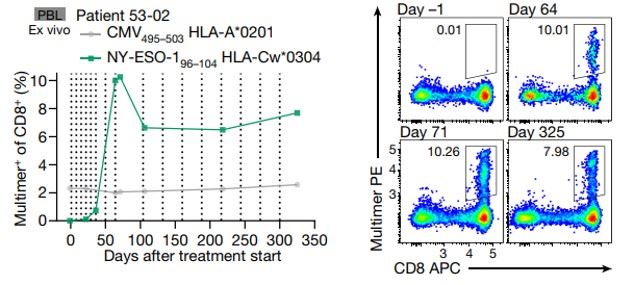
Fig.1. T-cell immunity induced by FixVac monotherapy. NY-ESO-1 96–104-specific Cw*0304-restricted CD8+ T cells analyzed by MHC I Dextramer® staining (left) and exemplary flow cytometry from different treatment time points (right). Negative control, cytomegalovirus (CMV)–pp65 multimer. Dashed lines indicate vaccinations.
Conclusions
- ≥75% of the analyzed patients showed polyclonal CD4+ and CD8+ T-cell responses against at least one of the vaccine antigens and mediated strong cytotoxic immunity
- Melanoma-specific MHC I Dextramer® reagents provide “the most informative assay for analyzing T-cell frequencies” and long-term immune monitoring following vaccination
- MHC I Dextramer® reagents are efficient tools for epitope identification in phenotypic characterization of T-cell immunity

