Get the case study as a PDF.
Immunophenotype and Persistence of CD4+and CD8+T Cells in the Phase I Trial of a TCR-T Cell Therapy Targeting MAGE-A4
Hong DS. et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial. Nature Medicine 2023. https://www.nature.com/articles/s41591-022-02128-z
Background
The intracellular melanoma-associated antigen A4 (MAGE-A4) is expressed in multiple solid cancers. Peptides of MAGE-A4 co-presented with human leukocyte antigens (HLA) are weakly recognized by natural T-cell receptors (TCR). This phase I trial examines the safety and dose range of afamitresgeneautoleucel(afami-cel), an autologous T cell therapy created by Adaptimmunethat expresses a high-affinity TCR targeting MAGE-A4 on HLA-A*02.
Study Description
Patients (38) diagnosed with 9 different solid tumor types received afami-celinfusion in 1 of 4 dose ranges. They were followed post-infusion to evaluate adverse events (AEs), dose-limiting toxicity (DLTs), persistence of the manufactured product, and clinical endpoints like partial response (PR), stable disease (SD), and progressive disease (PD). GMP-grade MHC Dextramer®reagents and flow cytometry were used to identify transduced CD8+and CD4+cells, assess transduction, determine phenotype composition of the manufactured product, and investigate the kinetics and long-term persistence of the therapy including population composition in post-infusion PBMC samples.
Results
- The ratio of transduced CD4+to CD8+T cells in the infused product varied from 16.16 to 0.03, mostly biasing CD4+cells. Median ratio was similar across clinical response (Figure 1A).
- afami-celpersistence peaked 7 days after infusion in most patients. Infused transduced cells were predominantly effector memory cells (EM) and terminally differentiated effector memory cells (EMRA).
- EMRA cells showed sustained presence over time, while the stem cell memory phenotype gradually increased (Figure 1B). Phenotype composition of the infused product was not associated with clinical response.
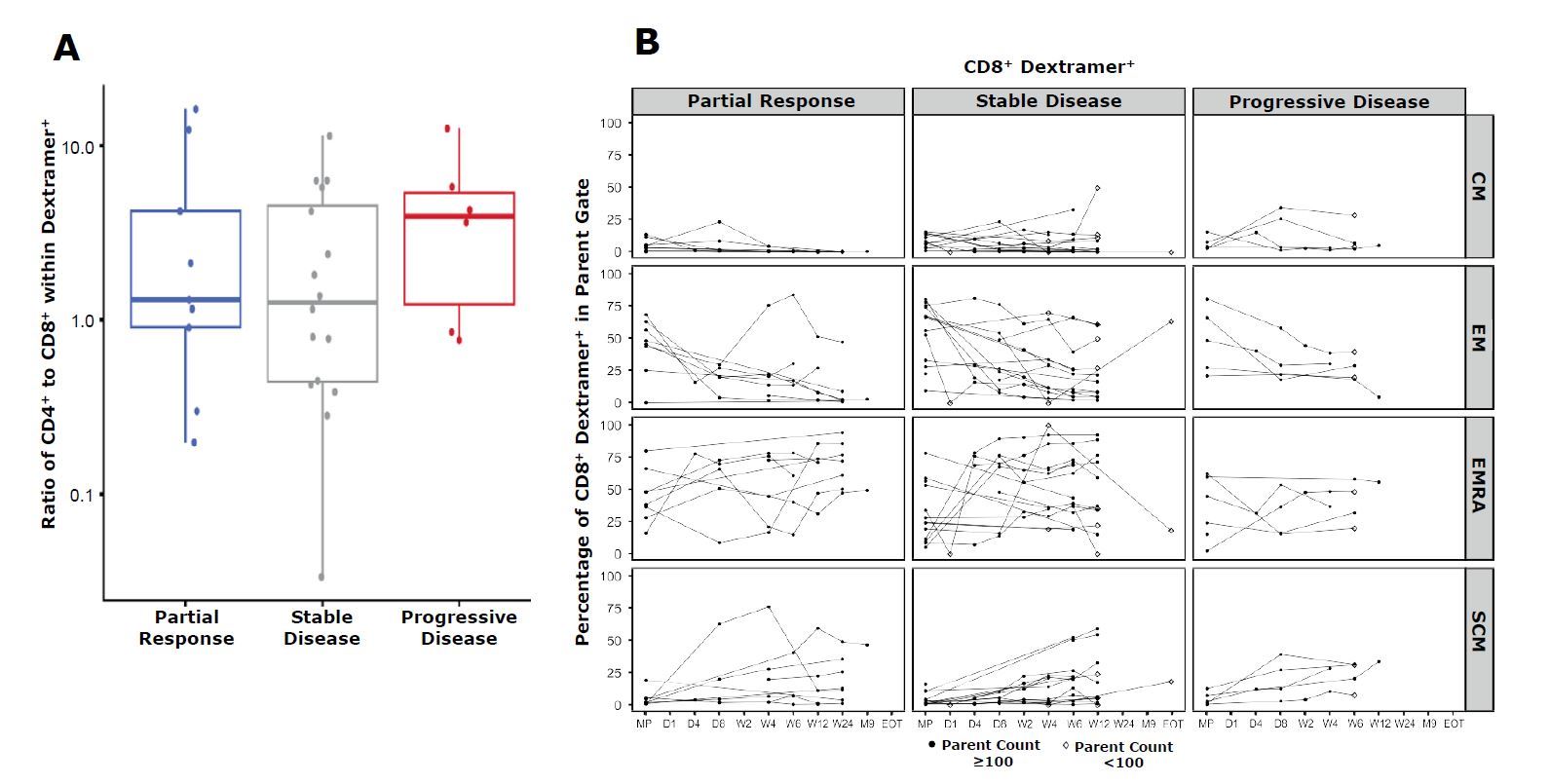
Fig.1: Composition of afami-celbefore and after infusion.
GMP-grade MHC Dextramer®reagents were used to assess A) CD4-to-CD8 ratio of the manufactured product, and B) cell type subsets before and after infusion
CM = central memory
EM = effector memory
EMRA = effector memory RA+ SCM = Stem cell memory
Conclusions
- afami-cell achieved clinically significant results for patients with multiple solid tumor types and showed a 94% disease control rate in patients with synovial sarcoma.
- CD4+and CD8+composition and phenotype profile of the manufactured product before and after infusion did not play a significant role in patient response to treatment.
- MHC Dextramer®(GMP) reagents offer high sensitivity, specific detection of engineered TCR-T cells, and enable the detailed characterization and longitudinal evaluation of a manufactured T cell therapy product.

