Get in Touch with our Immune Monitoring Experts
Reducing Risks of CMV Infection in Post-transplant Patients
- Precise monitoring: Rely on accurate detection and quantification of CMV-specific CD8+ T cells in transplant patients.
- Sensitive detection: Designed for high-avidity binding, Dextramer® technology ensures you detect even low-abundance cell populations.
- High signal-to-noise ratio: Each Dextramer® molecule carries multiple fluorophores that generate unambiguous signals and low background.
- Guide therapy decisions: Robust and reproducible results from whole blood samples can be helpful to avoid unnecessary antiviral treatment.
- For IVD use: The Dextramer® CMV Kit is available for in vitro diagnostic use in the EU and the US and includes appropriate negative controls.
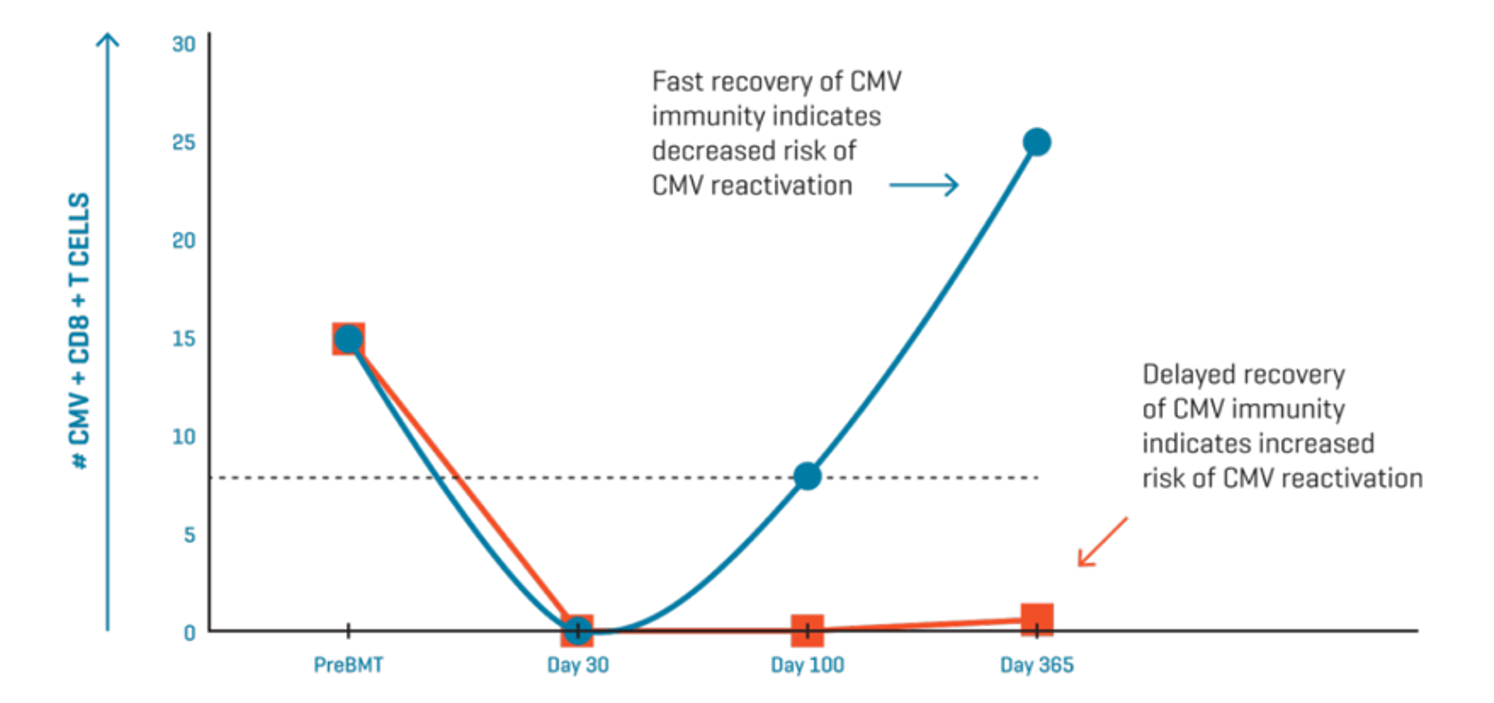
Cytomegalovirus (CMV) is a common infection with a high seroprevalence in human populations. Transplant patients are particularly susceptible to morbidity and mortality from infection because of treatment-related immunosuppression. Therefore, it is essential to monitor the CMV-specific immune status and risk of CMV reactivation in adult HSCT recipients following immunosuppression.
Immudex has developed the Dextramer® CMV Kit for in vitro diagnostic use in the EU and the US. Featuring a panel of CMV-specific Dextramer® reagents, the Dextramer® CMV Kit accurately enumerates CMV-specific CD8+ T cells in whole blood samples via flow cytometry.
"I have already recommended Immudex reagents to current and former colleagues because I am satisfied with the quality of the products and the customer support."
- Scientist, Pharmaceutical Company

