- Introduction to Potency Assays for TCR-T Cell Therapies
- Cytokine production following stimulation with Xynapse™-T - IFN-γ, TNF-α, and IL-2
- Enrichment of TCR-engineered T cells following stimulation with Xynapse™-T
- Biomarker expression patterns of TCR-engineered T cells activated with Xynapse™-T
- Evaluation of Xynapse™-T for a second TCR-T cell therapy product
Potency Testing of TCR-T Cell Therapies
Potency Assays Required to Evaluate TCR-T Cell Therapies
T cell receptor therapy has great promise for the treatment of solid tumors and in 2024 the U.S. FDA approved the first therapy for solid tumors based on TCR engineered T cells derived from the patient’s own T cells. The therapy named afami-cel is approved for the treatment of advanced synovial sarcoma and it is hopefully only the first example of many novel therapies to come.
Multiple T cell receptor therapies are currently in development for treatment of other cancer indications. Quality control of autologous cell therapy drug products derived from the patient’s own immune cells presents unique challenges distinct from other drug modalities. This is due to the complexity of living cells with multiple effector functions and the variable nature of patient-specific starting material.
An important part of the successful launch of a new cell therapy is the development of validated potency assays for release testing of cell products. These assays measure biological activities of the drug product which, per U.S. FDA guidance, must accurately reflect the mechanisms of action of the product to demonstrate its efficacy and safety1 and ideally correlate with clinical response.
The Immune Synapse at the Heart of Potency Assays
T cell receptor therapy is based on engineered T cells with TCRs designed to recognize a cancer specific epitope presented by a defined HLA. Thus endowed, the TCR-T cells are able seek out cancer cells presenting the cognate pMHC and kill them. Central to this activity is the formation of immune synapses between the TCR-T cells and antigen presenting cells (APC) and cancer cells which will trigger activation of the TCR-T cells leading cytokine secretion, clonal expansion and cytotoxic activity towards cancer cells specifically.
For this reason, potency assays for TCR-T cell products typically monitor the downstream effects of immune synapse formation in vitro between the TCR-T cells and antigen-presenting cells (APCs) loaded with the cognate peptide epitope. During drug development multiple assays are used to assess T cell activation, such as cytokine production assays, T cell expansion and cytotoxic activity. These biological effects are correlated and often a single or few surrogate markers can be selected for potency testing of commercially produced drug products (typically IFN-γ production).
APC-Derived Biological Variability
However, assays based on living APCs are intrinsically associated with high variability. In addition, their preparation and maintenance are labor-intensive, including cell culture, peptide loading, and validation. Furthermore, variable cell conditions, proliferation rates and risk of infections lead to unpredictable timelines. Delays in product release testing that can result from that inconsistency are critical for the terminally ill patients waiting for their treatment.
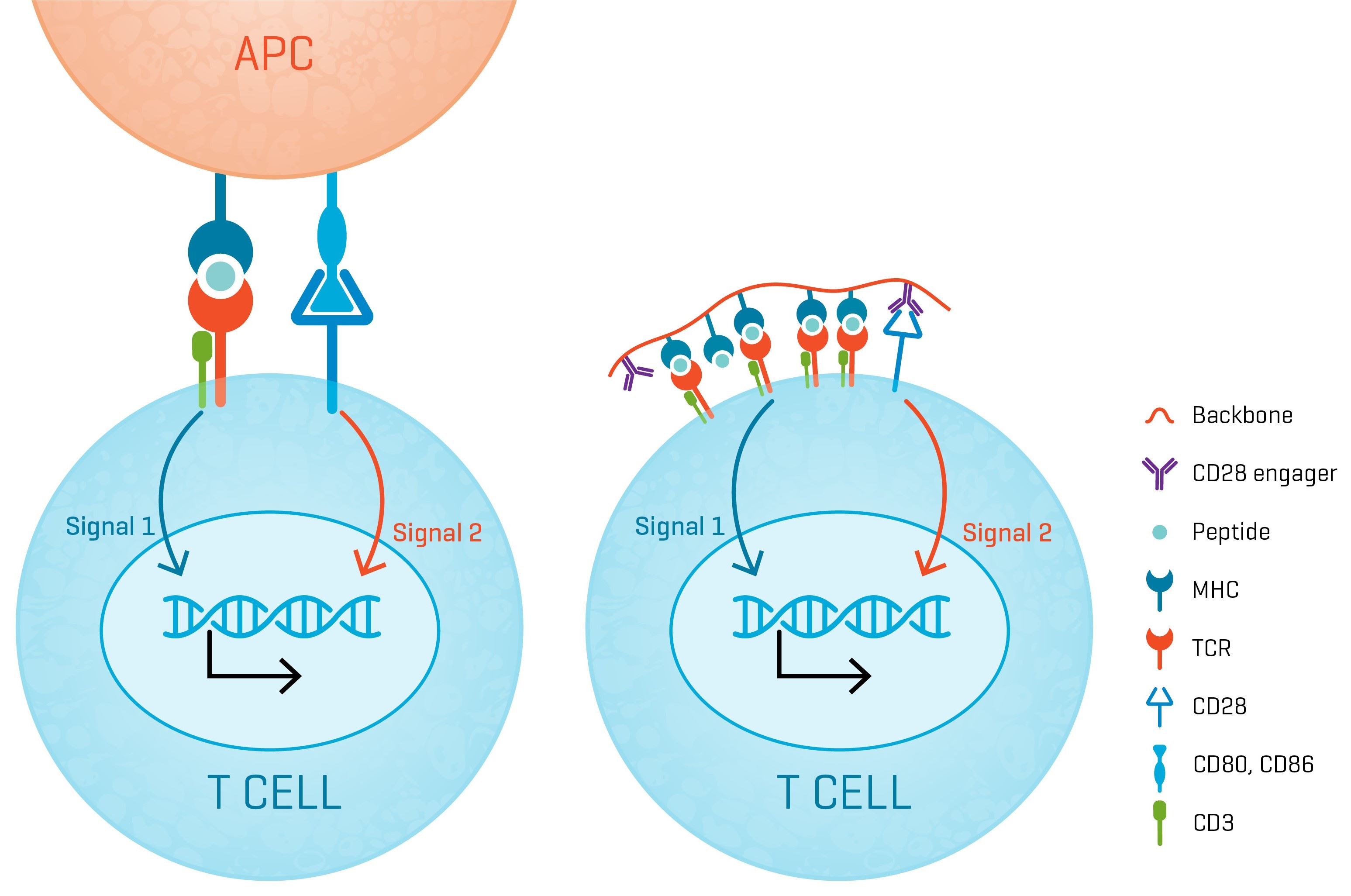
A Robust Mimic of the Immune Synapse
XynapseTM-T reagents are synthetic antigen-presenting molecules that eliminate the variability of working with APCs, offering significant time, labor, and standardization advantages. They consist of a polymeric backbone presenting peptide-MHC complexes and CD28 engagers to mimic the two-signal T cell activation by APCs.
Faster and More Flexible Potency Assays using Xynapse™-T Reagents
Ready-to-use XynapseTM-T reagents are produced rapidly and can be ordered with the peptide-MHC complex of choice, enabling design of highly standardized antigen-challenge assays.
Substituting APCs with synthetic XynapseTM-T molecules offers great flexibility in the planning and fast execution of potency assays, while the uniformity in performance ensures that results are reliable and comparable.
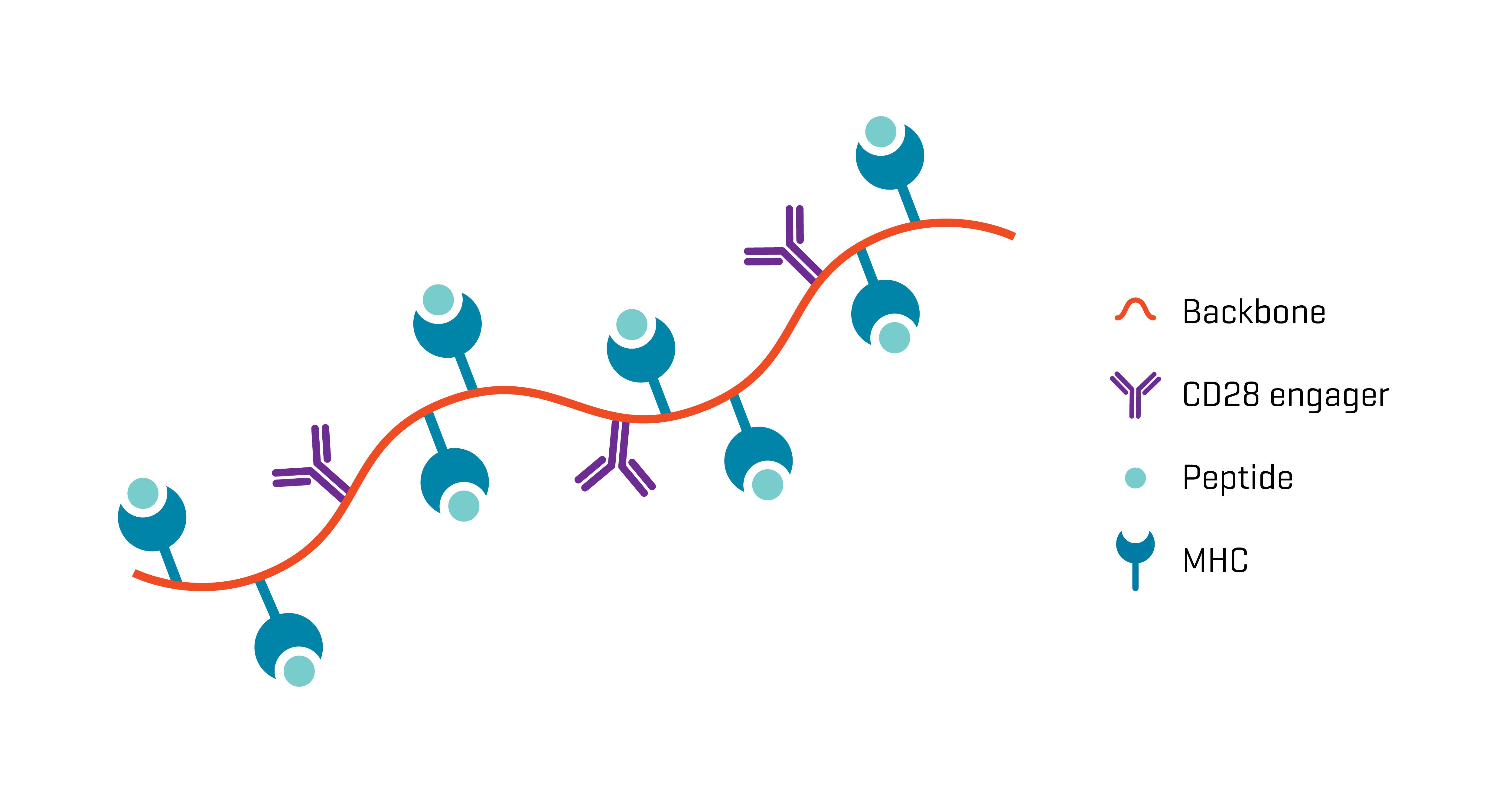
Are Xynapse™-T Reagents a Reliable Substitute for APCs?
To serve as proxy for peptide-pulsed APCs, XynapseTM-T reagents must demonstrably mimic their activation power. The following data were collected in collaboration with a partner comparing XynapseTM-T reagents with their APC counterpart and an αCD3 & αCD28 T cell activation reagent.
Stimulation of TCR-Engineered T Cells with XynapseTM-T Reagents is Antigen-Specific and αCD28-Dependent
TCR-engineered T cells with specificity for a defined cancer epitope and their wild type parental cells were co-cultured with XynapseTM-T reagents including various controls, an αCD3 & αCD28 T cell activation reagent, or APCs pulsed with the cancer epitope. After a 6-hour incubation, the proportion of TCR-engineered T cells producing the cytokines IFN-γ, TNF-α, and IL-2 were measured by flow cytometry.
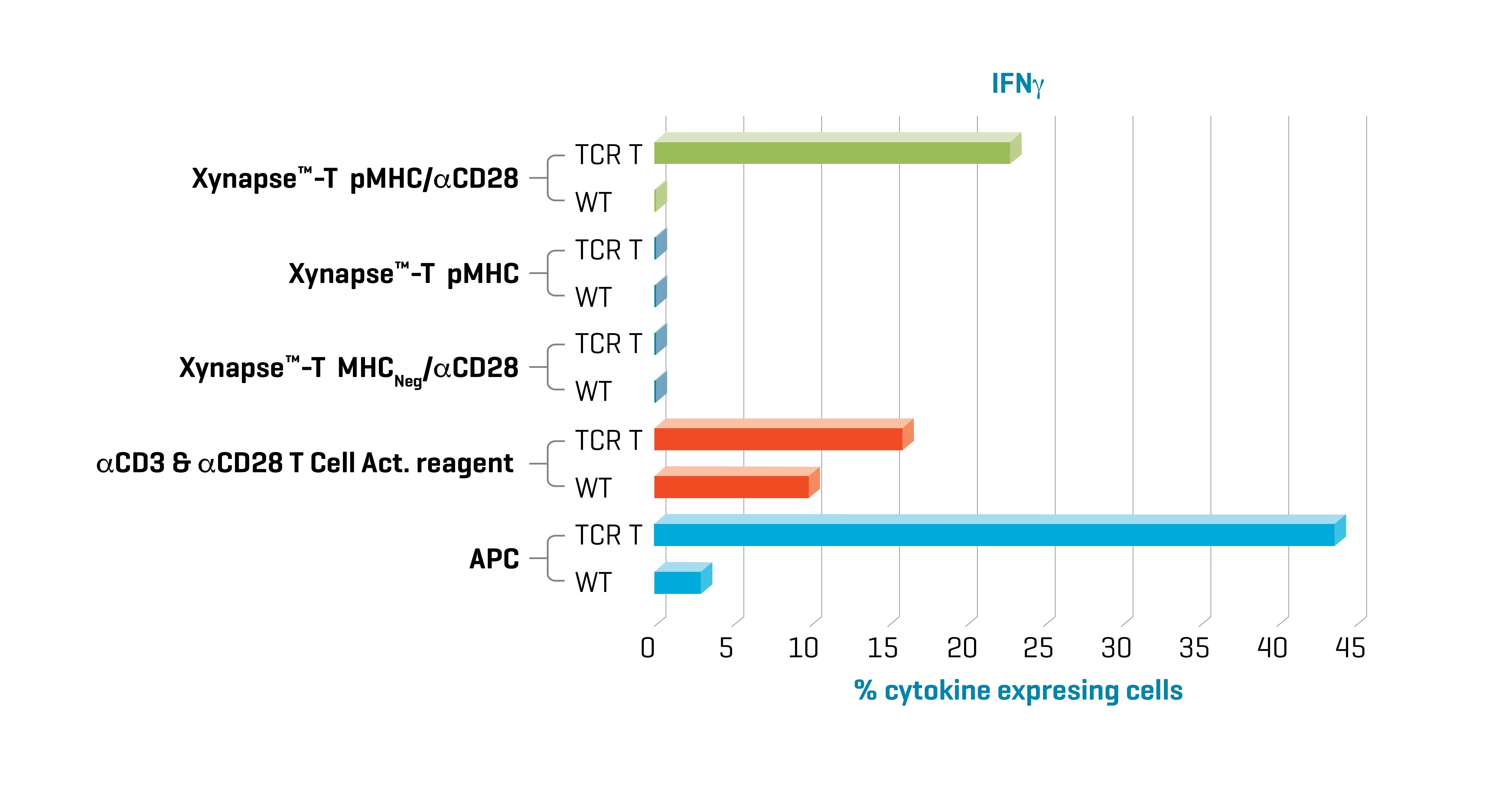
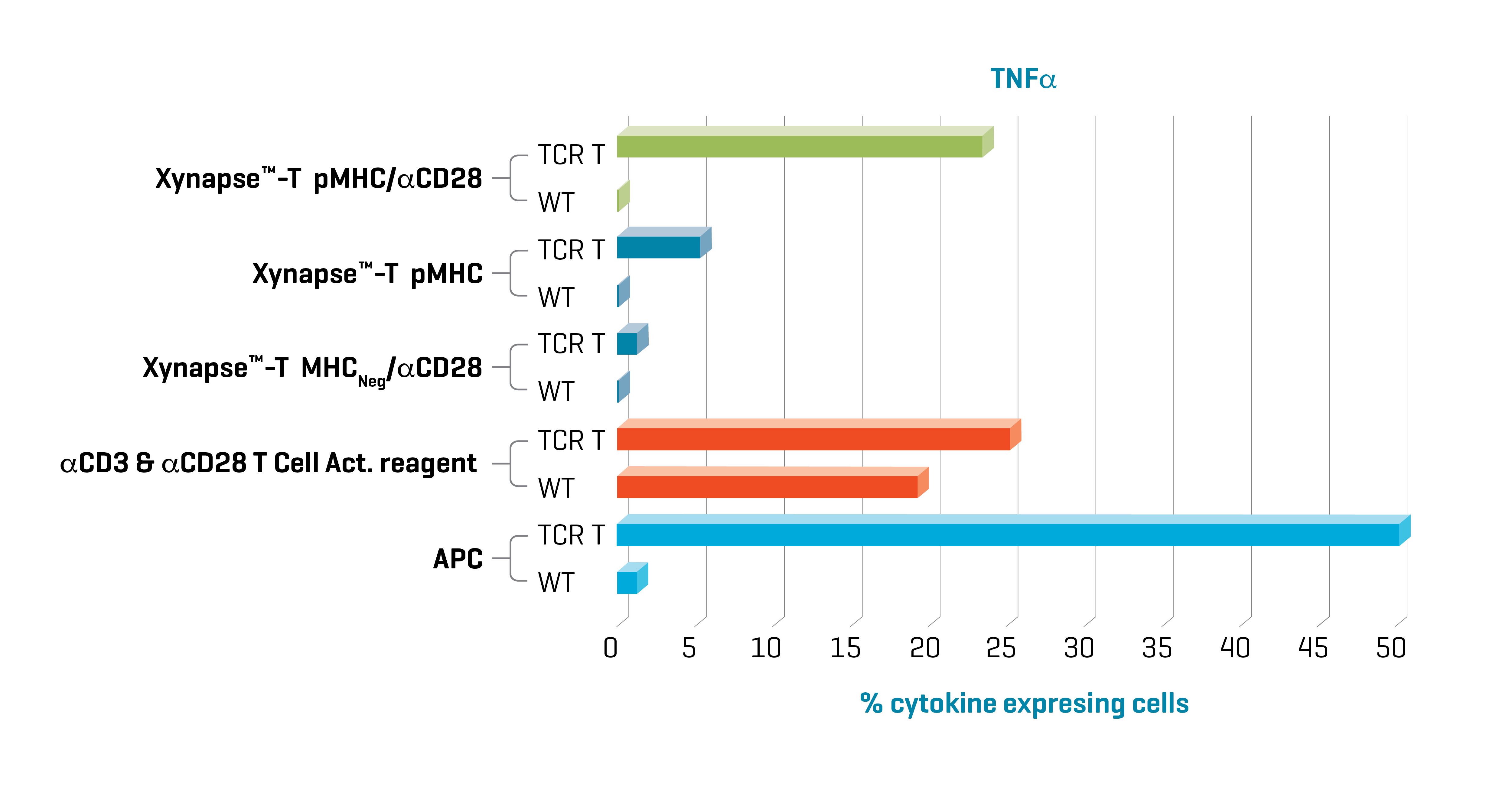
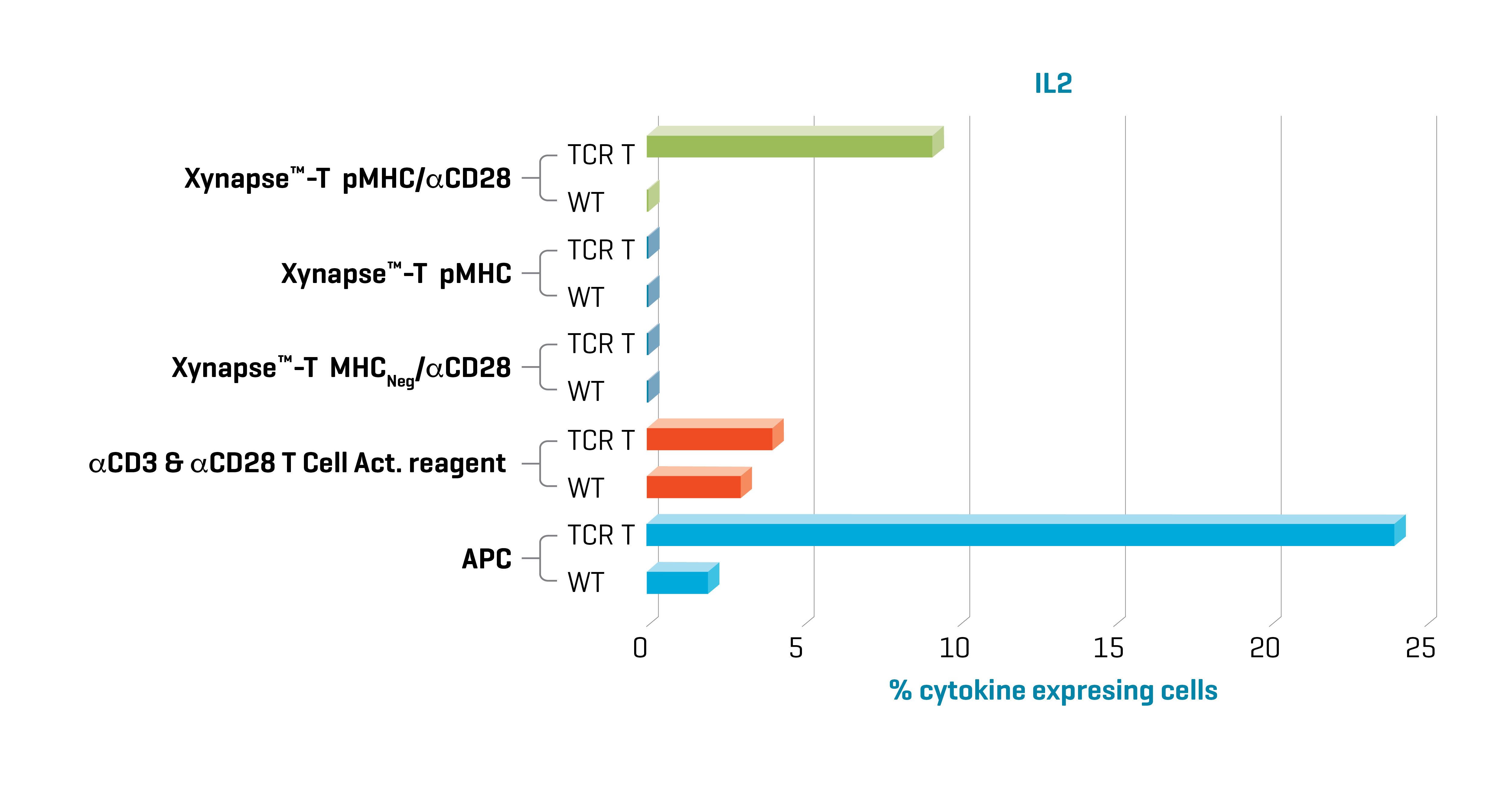
Figure 1. Xynapse™-T reagents stimulate TCR-engineered T cells to produce cytokines and upregulate activation surface markers (data not shown) in an antigen-specific and CD28 dependent manner. Data kindly provided by collaborator.
Induction of cytokine production with XynapseTM-T and APCs was specific to TCR engineered cells. Furthermore, cytokine production with XynapseTM-T was dependent on the presence of the CD28 engager on the polymer backbone and induction did not occur with a XynapseTM-T reagent displaying an irrelevant peptide (MHCNeg). In contrast the αCD3 & αCD28 T cell activation reagent also activated the parental wild type cells because it is designed to activate all T cells irrespective of antigen specificity via interaction with CD3.
XynapseTM-T Reagents Efficiently Expand and enrich TCR-Engineered T Cells
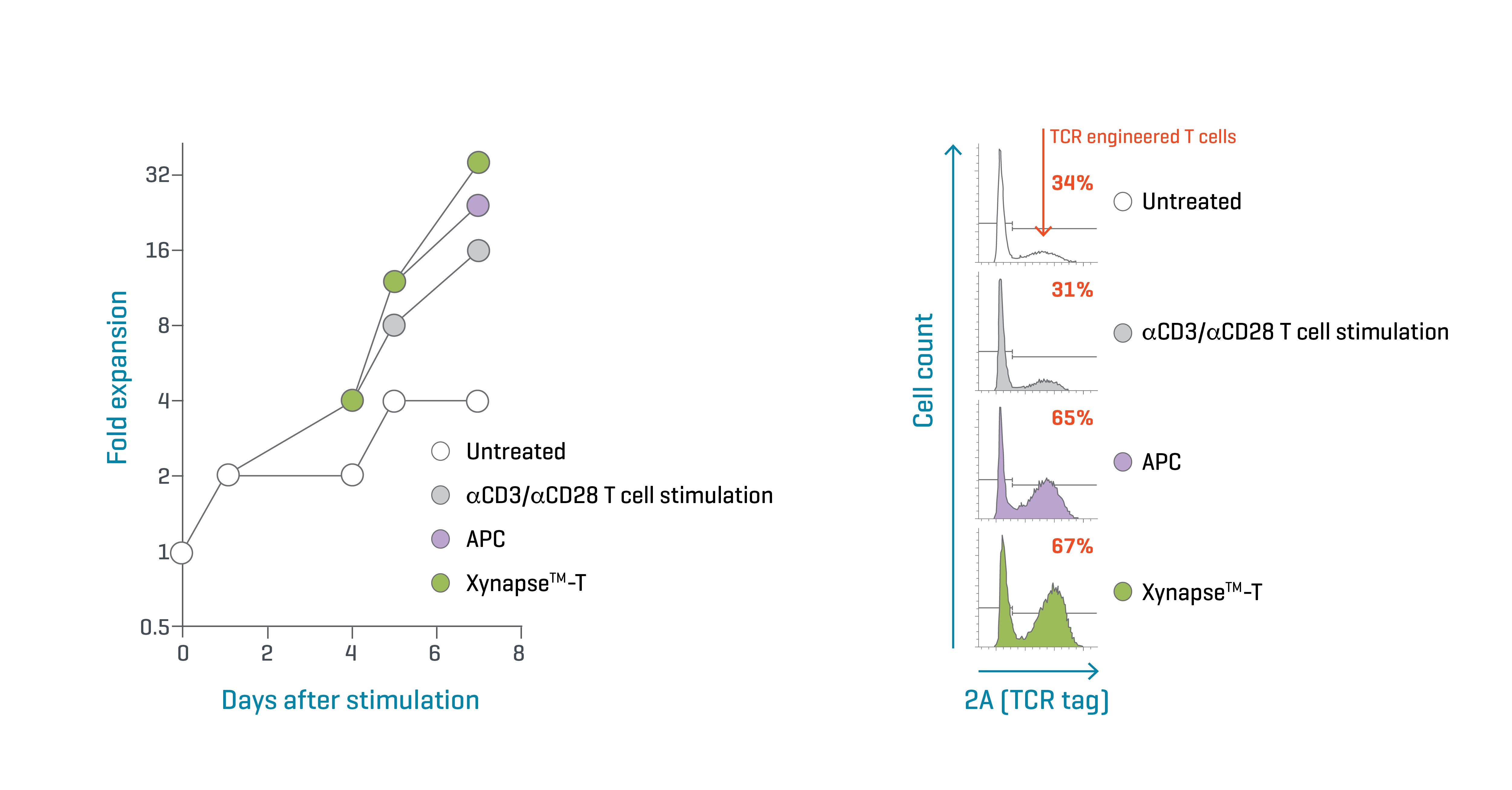
Figure 2. Xynapse™-T reagents efficiently expand and enrich TCR-engineered T cells. Data kindly provided by collaborator.
T cells transduced with a cancer-specific TCR were expanded with XynapseTM-T, a αCD3 & αCD28 T cell activation reagent, APCs loaded with the agonistic peptide, or untreated. Over 7 days, the proliferation rate of the cells was assessed at 4 timepoints (Figure 2, left). On day 7, the cultures were examined by flow cytometry to determine the proportion of TCR-engineered cells by detecting a tag built into the transduced construct (Figure 2, right).
Because the αCD3 & αCD28 T cell activation reagent activates all T cells in a culture, the proportion of TCR-modified cells did not change during expansion compared to the untreated culture. In contrast, the peptide-pulsed APCs and XynapseTM-T reagents stimulated proliferation of T cells expressing the engineered TCR. As a result, they were significantly enriched in culture, doubling in proportion in 7 days.
Activation with XynapseTM-T Reagents Resembles Activation by APCs
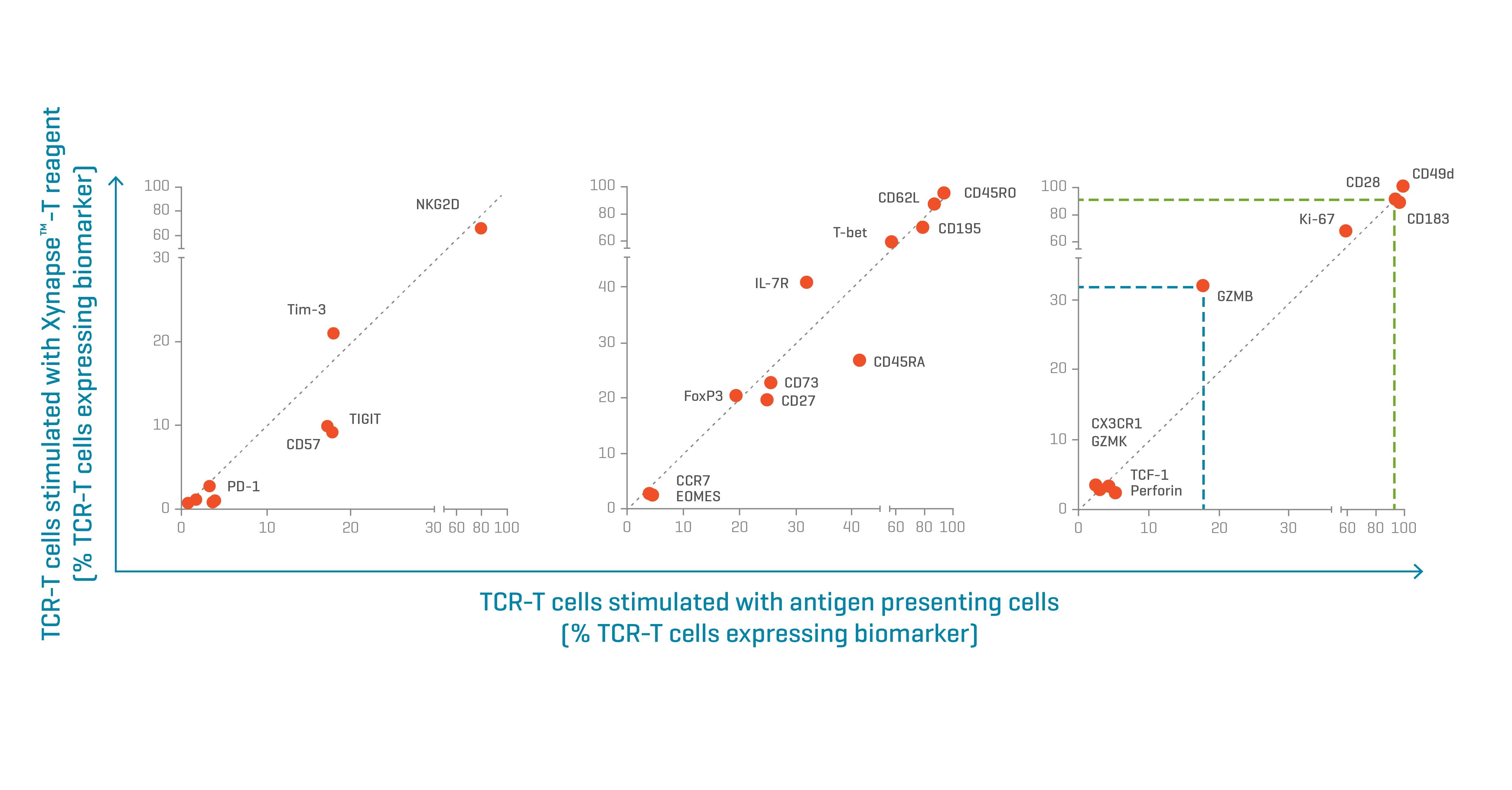
Figure 3. Biomarker expression patterns of TCR-engineered T cells activated with Xynapse™-T reagents resemble those of cells activated by peptide-pulse APCs. Biomarkers with similar expression levels between the two treatments align on the diagonal of each graph. Data kindly provided by collaborator.
To evaluate the effect of expansion on cell phenotype, TCR-engineered T cells were stimulated with XynapseTM-T reagents or peptide-pulsed APCs and cultured for 12 days. Following incubation, the stimulated TCR-engineered T cells were analyzed by flow cytometry with extended characterization panels to compare the phenotypes resulting from the two treatments.
The cells treated with XynapseTM-T reagents showed very similar biomarker expression patterns to those stimulated by APCs. The proportion of cells expressing any given biomarker aligned between the two activation methods (Figure 3). Only a few markers, like Granzyme B (expressed in a higher proportion of the XynapseTM-T treated cells) showed differential expression.
Evaluation of XynapseTM-T Reagents for a Second TCR-T Cell Therapy Product
Finally, a XynapseTM-T reagent was produced and tested for a second TCR-T cell product that is specific for an MHC displaying a different cancer epitope. TCR-T cell activation was evaluated by monitoring cytokine production by flow cytometry.
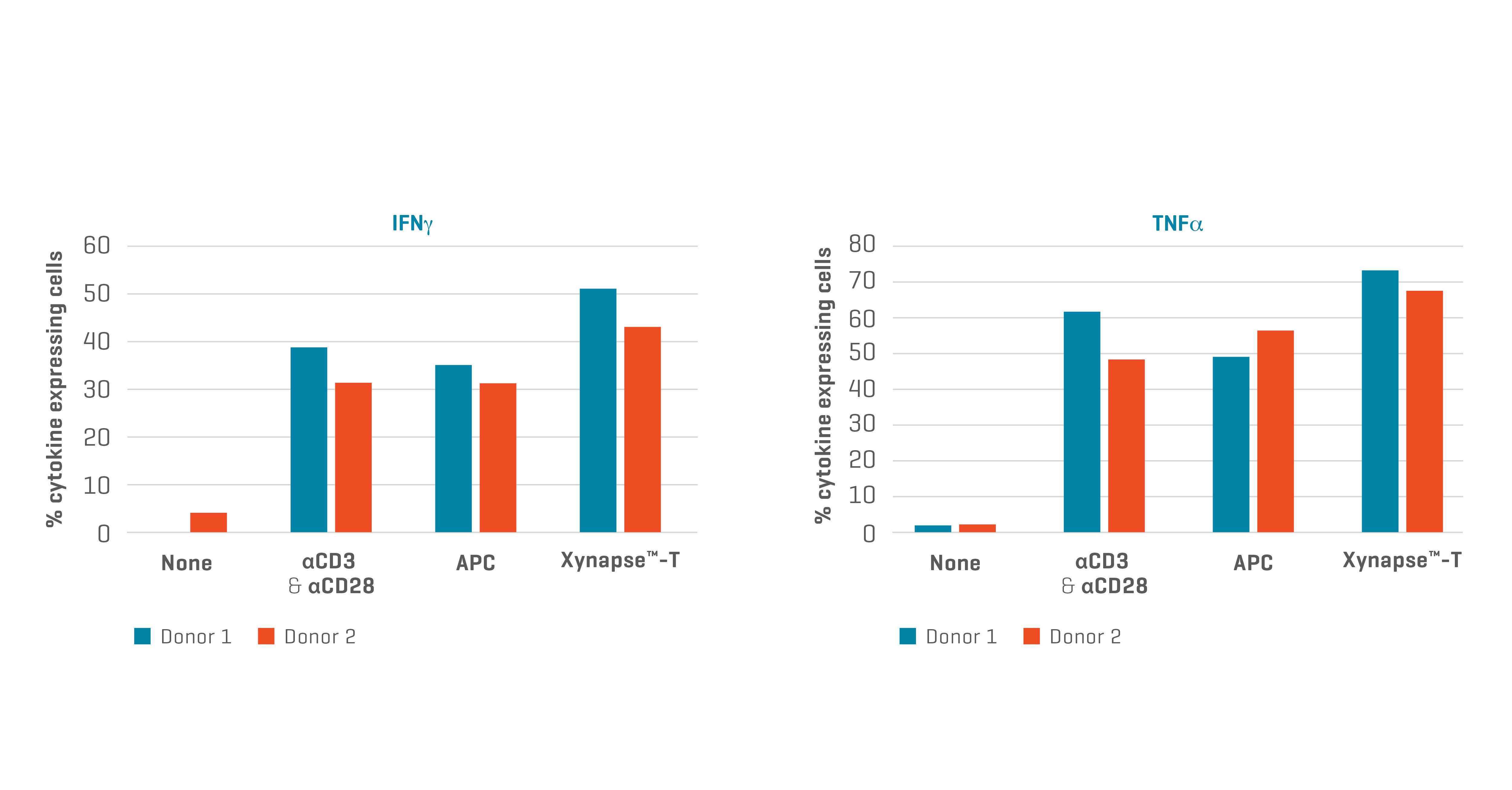
Figure 4. XynapseTM-T reagents stimulate TCR-engineered T cells to produce cytokines. Data kindly provided by collaborator.
Activation of this second TCR-T cell product with XynapseTM-T appears as efficient as that achieved with αCD3 & αCD28 T cell activating reagents and antigen presenting cells (APCs). XynapseTM-T technology therefore appears to be generally applicable as a substitute for APCs for development of T Cell Receptor Therapy.
Summary: XynapseTM-T Reagents Can Substitute for APCs in Potency Testing
The above presented results show that XynapseTM-T reagents deliver the same activation and expansion power of peptide-pulsed APCs, without the extensive preparation time and variability inherent to live cells.
Replacing APCs with XynapseTM-T reagents streamlines assay design and offers great flexibility in planning and execution of experiments. These reagents can be implemented in multiple stages of drug development from TCR discovery to functional characterization of engineered T cells, and potency assays for release testing of commercially produced cell products.
The significant enrichment of engineered T cells that can be achieved with XynapseTM-T could potentially be exploited to generate more potent drug products especially with donor cells that are not genetically modified efficiently.
TCR-T Release Testing
Discover how MHC Dextramer® technology supports release testing of TCR-T cell therapies.
Xynapse™ Reagents
Learn more about the product and order Xynapse™ reagents for your pMHC of interest.
Xynapse™ Product Flyer
Explore how Xynapse™ reagents can substitute APCs in potency tests and antigen challenge assays.
Xynapse™-T Protocol
Review the protocol for antigen-specific stimulation of T cells using Xynapse™-T reagents.
References
¹ U.S. Food & Drug Administration. 2011. Guidance for industry: Potency tests for cellular and gene therapy products.

