Get the case study as a PDF.
Exploring Neoantigen Cancer Vaccines in Combination with Immune Checkpoint Inhibitors Using MHC Dextramer®
Salavatori E. et al. Neoantigen cancer vaccine augments anti-CTLA-4 efficacy. https://doi.org/10.1038/s41541-022-00433-9
Background
Immune checkpoint inhibitors (ICI) based on anti-CTLA-4 (αCTLA-4) and anti-PD1 (αPD1) are being tested in combination with different therapeutic approaches, including other immunotherapies such as neoantigen cancer vaccines (NCV). In this study, Salvatori et al. investigated the effect of different therapeutic combinations of ICI with personalized DNA vaccines expressing neoantigens (C20) and delivered by electroporation (EP) in CT26 cancer-murine model. MHC Dextramer® reagents were applied to in-depth-characterize of the T-cell response to the tested treatment combinations.
Study Description
Balb/c mice were subcutaneously inoculated with 1x106 CT26 in the right flank. Mice were immunized with C20 on days 7, 14, and 21 and received ICI therapy on days 6, 13, and 20 (Fig. 1a). Researchers measured tumor growth twice a week for tumor growth studies. To identify immune correlates with the antitumor activity observed against established CT26 tumors, neoantigen-specific T-cell immune responses were analyzed in intratumoral lymphocytes and compared to splenocytes on day 20. For the flow cytometry assay, lymphocytes were harvested, single cells prepared, and stained with different antibody panels, including MHC Dextramer® (H-2Dd/SGPSYATYL).
Results
Tumor regression was observed in 100% of mice treated with αCTLA-4 and vaccinated with C20, and mice remained tumor-free for more than 250 days (Fig. 1b). A statistically significant increase of neoantigen-specific T cells was detected in both tissues of C20 vaccinated mice and those treated with αCTLA-4, but not with aPD1 (Fig. 1c). CTLA-4 is expressed by regulatory T cells, and it has been shown that αCTLA-4 can reduce this population in mice. In this experimental setting, a significant increase in the neoantigen-specific CD8+/Treg ratio was observed (Fig. 1d).
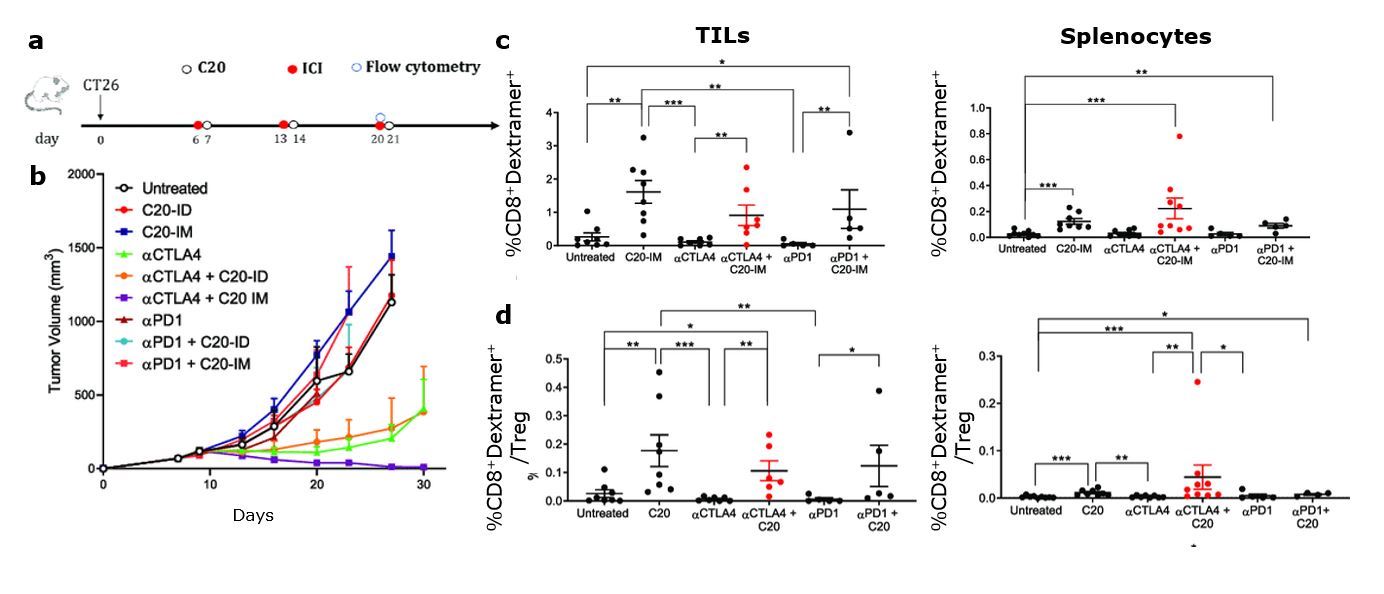
Fig. 1: Combined αCTLA-4/C20 treatment, therapeutic effects, and neoantigen specific immunological response. a) Therapeutic approach combining treatment with αCTLA-4 and C20 vaccine. b) Tumor volume measurement c) Frequency of neoantigen-specific response measured using the specific MHC Dextramer® (H-2Dd/SGPSYATYL) gated on CD45+CD3+CD8+ in live cells. d) Treg populations gated on CD45+CD3+CD4+ in live cells and expressed as ratio with Dextramer®-positive cells.
Conclusions
- The use of Dextramer® reagents showed that effector neoantigen-specific immune responses were enhanced in the periphery as well as in the tumor.
- The data support the use of NCV delivered by DNA electroporation with αCTLA-4 as a new protocol for clinical testing.

