Get the case study as a PDF.
Stringent identification and enumeration of a transient TCR T cell product for in vivo antitumor activity assessment
Background
Virally transduced T cells are often infused in large numbers to treat solid tumors, risking toxicity and insertional mutagenesis. mRNA gene transfer by electroporation reduces those risks by obviating viral vectors and inducing T cells to only transiently express therapeutic receptors. While effective, multiple doses are needed to achieve the desired effect. Looking to minimize repeat infusions, this study demonstrates that incubating T cells with a G9a/GLS inhibitor prior to electroporation boosts their antitumor activity
Study Description
The researchers engineered T cells to transiently express a TCR targeting hepatitis B virus envelope S183-191 (~72 h), having treated some with a G9a/GLS inhibitor during the expansion protocol. After extensive in vitro genomic, proteomic, metabolic, gene expression, and cytotoxic assessments, they conducted an in vivo antitumor activity experiment with an orthotopic xenograft mouse model of hepatocellular carcinoma (HCC).
The mice were injected with human liver carcinoma cells expressing luciferase to enable live imaging. 7 days after, the mice received a series of 7 infusions (every 2 days) of either no cells, T cells, T cells exposed to the G9a/GLS inhibitor, or transient TCR T cells treated or not treated with the inhibitor. The bioluminescence of the carcinoma cells was imaged over the course of the experiment. To consistently infuse the same number of cells across all treatments, “cells were more stringently analyzed” for TCR expression using HLA-A*201-HBV envelope 183-91-PE Dextramer® Reagent.
Results
Compared to all other treatment groups, mice that received the TCR-T cells treated with the G9a/GLS inhibitor showed a slow rise in bioluminescence (total photon flux; Figure 1) throughout the 22 days of observation, indicating restricted tumor growth. Other experiments in the study suggest that the boosted cytotoxicity is due to increased granzyme expression, which may explain why the mouse group that received G9a/GLS inhibitor-treated T cells also showed restricted tumor growth until day 20.
The researchers also showed that treating CAR-T and NK cells with the G9a/GLS inhibitor increased their cytotoxicity.
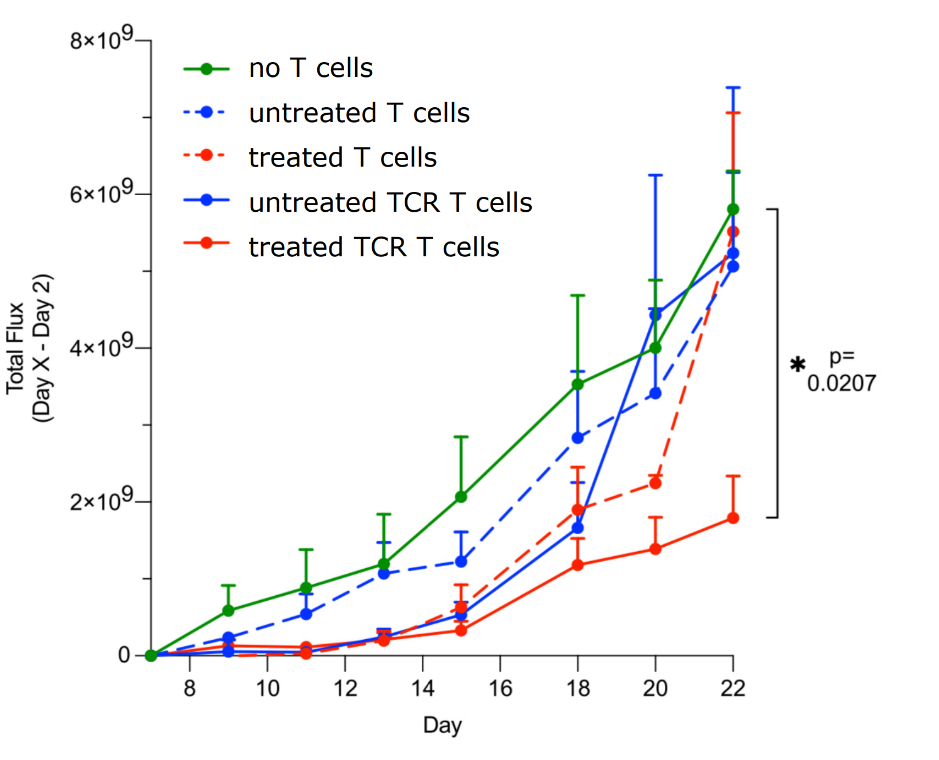
Fig.1: Restricted tumor growth in a xenograft mouse model of HCC after infusions of transient TCR-T cells treated with a G9A/GLS inhibitor. Custom Dextramer® reagents were used to accurately identify and dose the engineered TCR-T cells.
Conclusions
- Antitumor activity of transient TCR-T cells generated via mRNA gene transfer is improved when incubated with a G9a/GLS inhibitor prior to electroporation.
- G9a/GLS inhibition could be used to reduce side effects of adoptive cell transfer and reduce manufacturing costs by generating more potent engineered cells.
- Dextramer® reagents afford the sensitivity and specificity to identify and enumerate engineered T cells for accurate in vivo studies.

